Streamline Clinical Trials with Automated Protocol Workflow Support
We offer an innovative clinical trial software solution that offers protocol automation and workflow support. Designed specifically for the healthcare industry, our software empowers clinical trial teams to streamline processes, increase efficiency, and ensure compliance.
By automating protocol workflows, our solution revolutionises the way clinical trials are conducted, bringing numerous benefits to researchers, sponsors, and participants alike.

Key features
Automated Protocol Execution
Our software automates the execution of complex protocols, reducing manual errors and ensuring adherence to predefined processes. By automating data collection, patient enrollment, visit scheduling, and other protocol-related tasks, our solution minimizes administrative burden, freeing up valuable time for researchers to focus on critical activities.
Intelligent Task Assignment
With our software, you can intelligently assign tasks to team members based on their expertise, availability, and workload. The system optimizes resource allocation, ensuring that each task is assigned to the most suitable team member, enhancing collaboration and productivity.
Real-time Protocol Monitoring
Gain full visibility into your clinical trial's progress with real-time monitoring of protocol milestones and metrics. Our software provides comprehensive dashboards and reports, allowing you to track enrollment rates, protocol deviations, adverse events, and other key performance indicators. This real-time insight empowers you to make informed decisions and take proactive actions to keep your trial on track.
Compliance and Audit Readiness:
Our solution includes built-in compliance features, ensuring that your clinical trial adheres to regulatory requirements and industry standards. By automating data capture and documentation processes, our software minimizes the risk of compliance issues and facilitates easier audits, saving you time and resources.
Benefits and value
Enhance your research with automation
Our modules reduces manual effort, accelerates study timelines, and improves overall trial efficiency.
Usecases
By automating protocol workflows, our software reduces manual effort, accelerates study timelines, and improves overall trial efficiency.
Researchers can focus on critical tasks, resulting in faster trial completion and time-to-market for life-saving treatments.
Enhanced Data Accuracy
With automated data collection and reduced manual errors, our software improves data accuracy, reliability, and integrity.
This leads to more reliable outcomes, precise analyses, and confident decision-making, ultimately improving the quality of clinical trial results.
Improved Participant Experience
Our solution streamlines the participant journey, offering seamless communication, automated reminders, and personalised engagement.
Participants can easily schedule visits, access educational materials, and provide feedback, leading to higher retention rates and improved patient satisfaction.
Usecases
Streamline your research with Automation
Discover how our modules optimise clinical trials and academic research, enhancing data collection and participant comprehension.
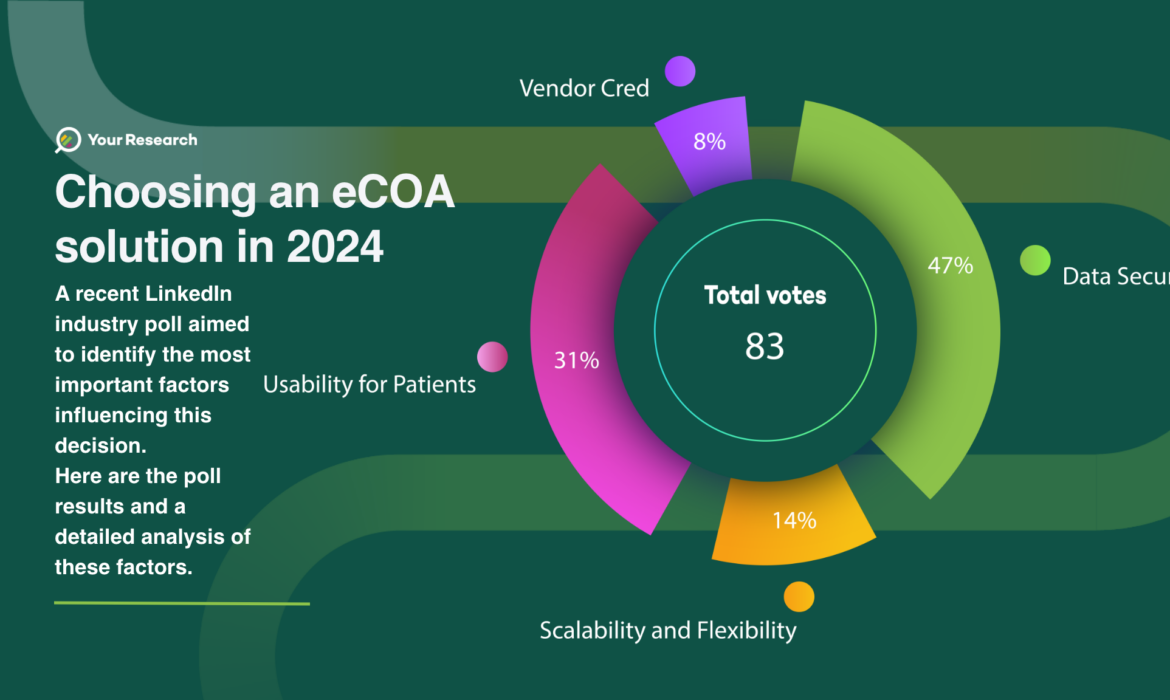
Choosing an eCOA solution in 2024
Technical specifications
Integrate, secure, compliant
Integration
Compliance
Usability
- System is compatible and integrates with existing technology infrastructure.
- Capable of integrating with other clinical trial systems such as electronic data capture (EDC) systems, electronic health record (EHR) systems, or laboratory information management systems (LIMS).
- Allows for seamless data transfer, which reduces duplicate data entry, and enables efficient data management across multiple systems.
- Has robust security measures to protect patient data, ensure data privacy, and meet regulatory requirements.
- This includes encryption of data, user authentication mechanisms, access controls, audit trails, and adherence to data privacy regulations: HIPAA (Health Insurance Portability and Accountability Act) and GDPR (General Data Protection Regulation).
- Complies with relevant data security standards and provide necessary documentation for regulatory audits. (GCP)
- User-friendly interface that is intuitive and easy to use.
- Designed to accommodate different types of users, including investigators, site staff, and patients.
- Provides clear instructions, logical data entry workflows, and features such as dropdown menus, checkboxes, and auto-population to simplify data entry.
- Includes support for multiple languages, accessibility for users with disabilities, and responsiveness across different devices and screen sizes.
Related modules
Along the participant journey
With Your Research, you have the flexibility to utilize stand-alone modules, integrate them with your existing software vendors, or connect them to other modules within Your Research to enhance the participant journey.
Enhance your research processes
Contact us and we will be happy to show you how Your Research makes a difference in conducting clinical research, making research more efficient and personalised for everyone involved.