Best in-class technology; unparalleled service
eCOA excellence
Your Research’s electronic Clinical Outcome solution transforms how clinical trials collect and manage patient-reported outcomes.
With an emphasis on real-time data capture, ease of use, and compliance, our eCOA system enables participants to provide accurate data directly from their devices.
This intuitive solution enhances data quality and engagement, delivering timely insights to sponsors and CROs while reducing administrative burdens and ensuring protocol adherence.
Immediate data capture from patients, caregivers, and clinicians, improving data accuracy and study responsiveness.
Simple, intuitive interface designed to boost compliance and participation, making it easy for patients to complete assessments.
Fully compliant with regulatory requirements, including 21 CFR Part 11, GDPR, and GCP, ensuring secure and reliable data handling.
Designed to emphasise the crucial role of human interaction in driving engagement and improving trial outcomes.
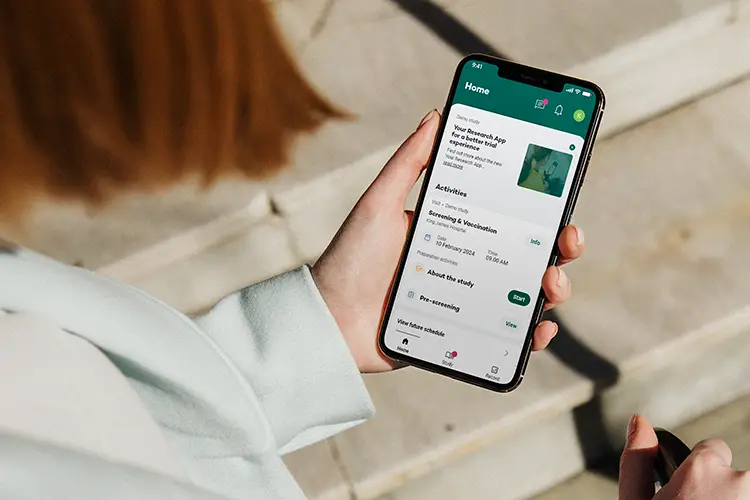
Key features
Seamless, secure, and engaging data collection
Leveraging both retrospective and prospective insights, our intelligent workflow guidance supports efficient and accurate trial management.
By providing an exceptional experience, timely notifications, and enhanced usability for participants, we effectively mitigate both study protocol adherence and patient retention risks.
Pre-validated or tailored questionnaires including real-time data validation checks and reminders to ensure completeness and accuracy of the clinical trial data.
Your Research employs smart participant retention methods to keep participants engaged and committed throughout clinical trials. These include personalized communication, flexible scheduling, gamification, and real-time feedback, all seamlessly integrated into our platform. By enhancing the participant experience and reducing administrative burdens for sites, Your Research ensures higher retention rates and more reliable trial outcomes.
eCOA includes questionnaires, scales, and diaries that can be tailored to the study's requirements and are designed to be user-friendly for accurate self-reporting.
Featuring a single user-personalized interface for study oversight, Your Research streamlines operations and ensures seamless integration with other systems through Single Sign-On (SSO) and API capabilities.
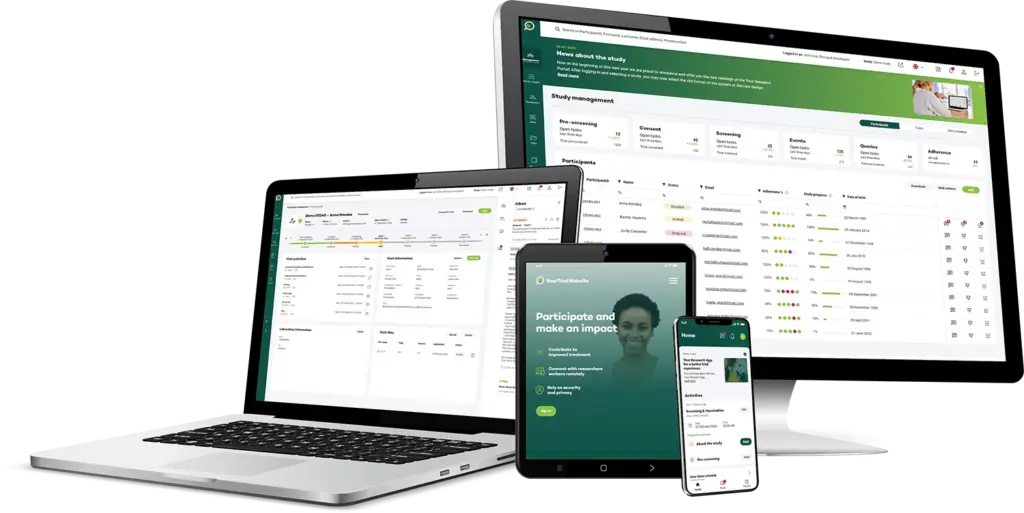
Types of Assessments
Your Research facilitates efficient data management, organisation, and analysis, enabling study teams to proactively access and analyse patient-reported data more easily.
Our services
Streamline service delivery to minimize risks and expedite startup
Minimising startup and delay risks is the goal of our services. Whether you prefer a hybrid or full self-service model, we are ready to assist with our expertise and experience, ensuring peace of mind.
Pre-validated questionnaires will save you time setting up your trial and data quality risks
Our network of translators will support with validated translation of questionnaires and communication information for participants.
Together with our technology, local partners and pre-defined documentation, we support you to have oversight into your IRB approval process.
Your Research provides pre-configured devices to patients for consistent performance and controlled study environments based on a hybrid or full provisioning model.
We provide 24/7 assistance to address technical issues and user inquiries, as well as training for clinical staff to ensure they are proficient in using Your Research.
Modules at a glance
Elevate the whole Participant Journey
Serving as the essential foundation, our Unified Protocol facilitates the seamless integration of both modules and third-party systems along the Participant Journey.
This foundational structure ensures comprehensive oversight and enables strategic foresight throughout the patient journey.





