Insights from Industry Poll: Key Factors in Selecting an eCOA Solution for Clinical Research
Introduction
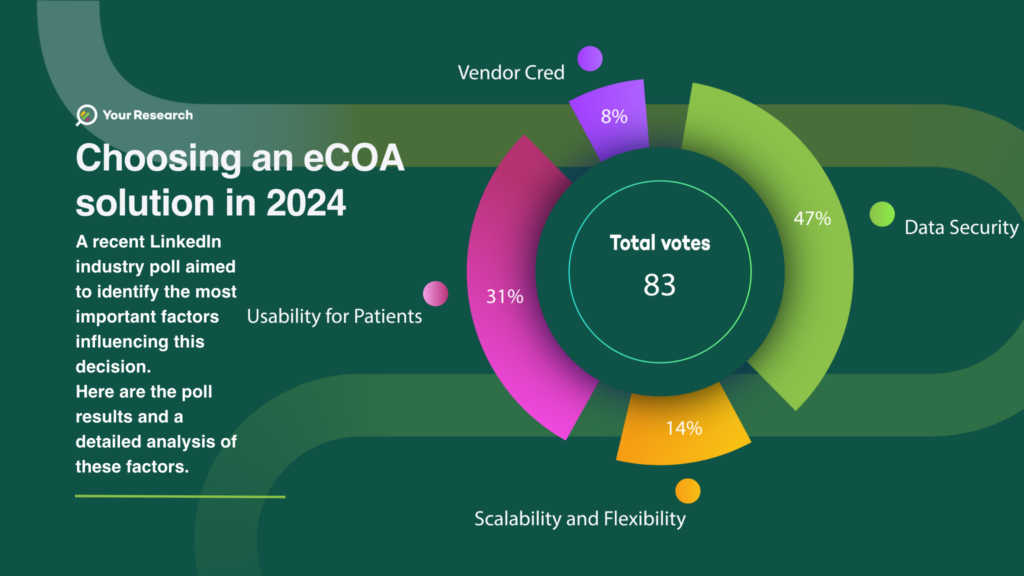
Selecting an electronic Clinical Outcome Assessment (eCOA) solution is a critical decision for organisations engaged in clinical research. A recent industry poll conducted on LinkedIn aimed to identify the most important factors influencing this decision.
The poll gathered insights from 83 professionals, revealing distinct priorities that drive the selection process. Here are the results:
- Regulatory Compliance, Validation, and Data Security: 30 votes
- User Experience and Accessibility: 20 votes
- Scalability and Flexibility: 9 votes
- Vendor Credibility and Service Offering: 5 votes
Detailed Analysis of Poll Results
 1. Regulatory Compliance, Validation, and Data Security
1. Regulatory Compliance, Validation, and Data Security
This factor emerged as the most critical, receiving the highest number of votes. It underscores the importance of ensuring adherence to regulatory standards such as FDA 21 CFR Part 11, EMA guidelines, and Good Clinical Practice (GCP) standards.
Key Considerations:
- Regulatory Adherence: Ensuring the eCOA solution complies with stringent regulatory requirements is essential to maintain the integrity and validity of the collected data.
- Data Security: Robust encryption methods, compliance with data privacy regulations (such as GDPR), and secure data backup procedures are paramount to protect sensitive patient information.
- Validation: Proper validation processes ensure that the eCOA system is reliable and produces accurate data consistently.
 2. User Experience and Accessibility
2. User Experience and Accessibility
The second most important factor is the user experience and accessibility of the eCOA solution. This emphasises the need for solutions that are easy to use and accessible to a diverse range of users.
Key Considerations:
- Intuitive Interface: The interface should be user-friendly, reducing the learning curve for users and minimising the risk of errors.
- Device Compatibility: The solution should be compatible with various devices, including smartphones, tablets, and desktop computers, to facilitate ease of use.
- Multilingual Support: Providing support for multiple languages ensures inclusivity and accessibility for global clinical trials
 3. Scalability and Flexibility
3. Scalability and Flexibility
Scalability and flexibility are crucial for accommodating the dynamic nature of clinical trials. This factor, though not the top priority, still plays a significant role in the selection process.
Key Considerations:
- Large-Scale Trials: The ability to scale the eCOA solution to handle large volumes of data and participants is vital for extensive clinical studies.
- Protocol Changes: Flexibility to adapt to protocol amendments without significant delays or costs is essential for maintaining the momentum of the research.
- Integration Capabilities: Seamless integration with other systems and platforms used in clinical research ensures a streamlined workflow and data consistency.
 4. Vendor Credibility and Service Offering
4. Vendor Credibility and Service Offering
Vendor credibility and the range of services offered, though receiving the fewest votes, remain important for ensuring a reliable and comprehensive eCOA solution.
Key Considerations:
- Established Vendors: Working with reputable and well-established vendors reduces risks associated with product reliability and support.
- Comprehensive Services: Services such as translations, device provisioning, and licensing support enhance the overall value of the eCOA solution.
- Customer Support: Reliable customer support and training services from the vendor are crucial for addressing issues promptly and ensuring smooth operation.
3 Other points to consider
1. Interoperability and Data Integration
The ability of an eCOA solution to integrate seamlessly with other systems used in clinical trials is crucial. Interoperability ensures that data can be easily shared, compared, and analyzed across different platforms, enhancing the overall efficiency and coherence of the trial processes.
Key Aspects:
- Integration with EDC Systems: Ensure the eCOA solution can integrate with Electronic Data Capture (EDC) systems to streamline data collection and management.
- Compatibility with eTMF and CTMS: The solution should be compatible with electronic Trial Master File (eTMF) systems and Clinical Trial Management Systems (CTMS) for comprehensive trial oversight.
- API Availability: Availability of robust Application Programming Interfaces (APIs) for custom integrations and data exchange.
2. Patient Engagement and Compliance
Enhancing patient engagement and ensuring compliance are critical for the success of clinical trials. An effective eCOA solution should offer features that facilitate patient participation and adherence to the trial protocol.
Key Aspects:
- Reminders and Notifications: Automated reminders and notifications for patients to complete assessments can improve compliance rates.
- Ease of Use for Patients: Features like clear instructions, user-friendly design, and minimal technical requirements make it easier for patients to engage with the solution.
- Support for Remote Monitoring: Capabilities for remote monitoring and support can help maintain patient engagement and compliance, especially in decentralized or hybrid trials.
3. Cost and Return on Investment (ROI)
Understanding the cost implications and potential return on investment of an eCOA solution is crucial for making an informed decision. Evaluating the total cost of ownership and the potential benefits can help justify the investment.
Key Aspects:
- Initial and Ongoing Costs: Consider the upfront implementation costs, as well as ongoing costs for maintenance, updates, and support.
- Cost Savings: Evaluate how the solution can reduce costs associated with data entry errors, monitoring, and paper-based processes.
- ROI Analysis: Assess the overall return on investment by considering improved data quality, faster trial completion times, and enhanced regulatory compliance.
Conclusion
The results of this poll highlight the multifaceted considerations involved in selecting an eCOA solution for clinical research. While regulatory compliance, validation, and data security stand out as the top priority, other factors such as user experience, scalability, flexibility, and vendor credibility also play significant roles.
By carefully evaluating these factors, organisations can select an eCOA solution that not only meets regulatory requirements but also enhances the efficiency and accuracy of their clinical trials.
About Us
Your Research is a rapidly expanding software company dedicated to supporting the clinical research industry in discovering new therapies and healthcare solutions. Our intuitive solutions guide participants, support researchers, and empower clinical teams.
For more information about our services related to this article, please click here.