eRegistration
E-registration refers to the electronic registration of trial participants. It is an alternative to traditional paper-based registration where participants complete registration forms on paper.
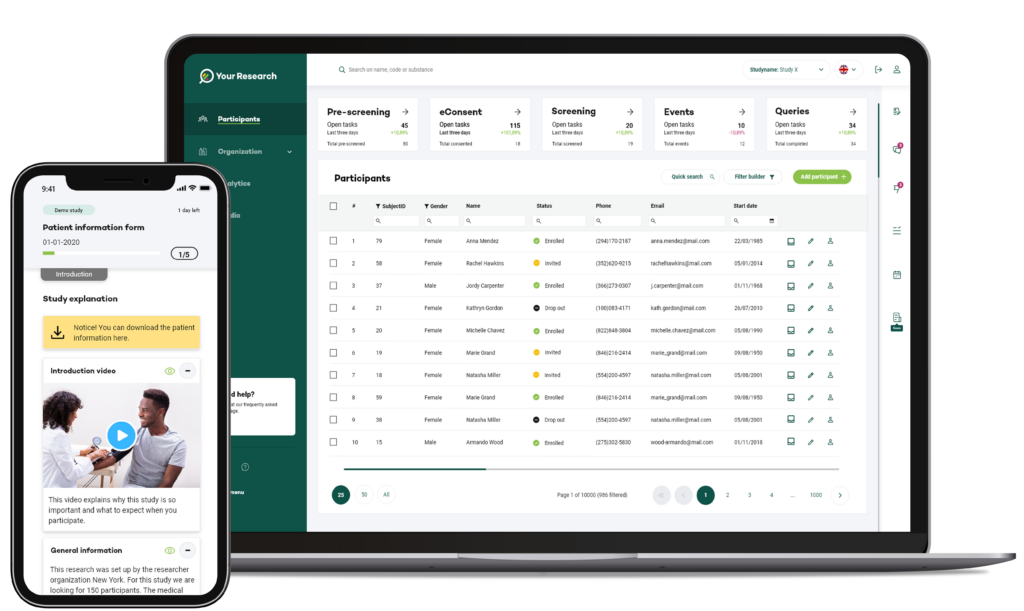
The Your Research eRegistration module within the Your Research Portal enables participants to register for a trial using electronic devices, such as tablets. This versatile system can be customised to gather a comprehensive range of participant data, including demographic information, medical history, and informed consent.
Key features
Set up your process
Experience the ultimate flexibility of our modular eRegistration module. Tailor the key features to your study protocol, ensuring a seamless and customised process that aligns perfectly with your research objectives.
Online Participant Registration
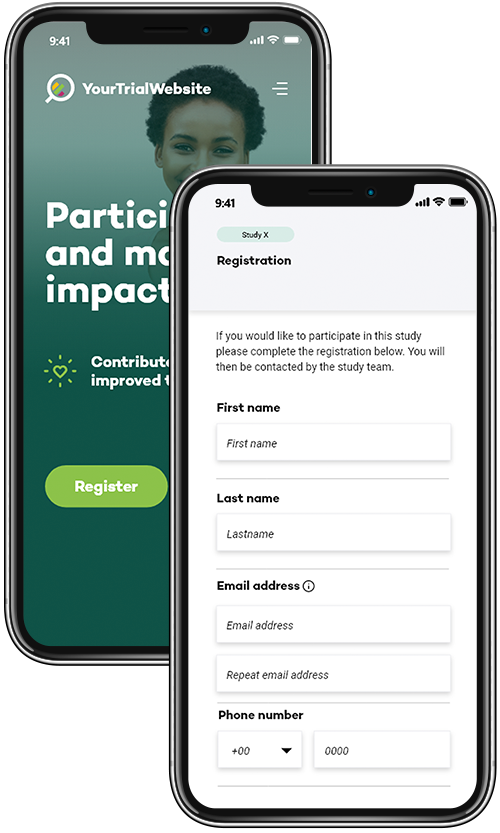
Your Research's eRegistration module enables participants to register for clinical trials through an online platform or portal.
Participants can provide their demographic information, medical history, and contact details electronically, eliminating the need for paper-based registration forms.
The online registration process can be accessed remotely, making it convenient for participants and allowing for a broader reach in recruitment efforts.
Automated Eligibility Screening
eRegistration system incorporates automated eligibility screening mechanisms.
Participants' responses and provided information can be assessed against predefined eligibility criteria in real-time.
The system can flag potential eligibility issues and provide immediate feedback to participants or research staff, streamlining the screening process.
This feature helps ensure that only eligible participants are enrolled in the study, saving time and resources by reducing the need for manual screening.
Consent Management
Consent management is a crucial aspect of eRegistration.
The system allows participants to electronically provide informed consent, typically through digital signatures or electronic consent forms.
Your Research eRegistration module includes interactive elements, such as multimedia content or questionnaires, to enhance participant understanding and engagement during the consent process.
The system securely stores and tracks consent documentation, ensuring compliance with ethical and regulatory requirements.
Consent management in eRegistration enhances participant convenience, streamlines the consent process, and improves documentation and accountability.
Benefits and value
Simplify the registration and enrolment process
eRegistration contributes to efficient participant recruitment, streamlined eligibility screening, and enhanced consent management in clinical trials.
The Your Research eRegistration module significantly simplifies the registration and enrolment process, improves data accuracy, and facilitates compliance with regulatory standards.
Online Participant Registration
eRegistration enables participants to register for clinical trials through an online platform or portal.
Participants can provide their demographic information, medical history, and contact details electronically, eliminating the need for paper-based registration forms.
The online registration process can be accessed remotely, making it convenient for participants and allowing for a broader reach in recruitment efforts.
Automated Eligibility Screening
The Your research eRegistration module incorporates automated eligibility screening mechanisms.
Participants’ responses and provided information can be assessed against predefined eligibility criteria in real-time.
The system can flag potential eligibility issues and provide immediate feedback to participants or research staff, streamlining the screening process.
This feature helps ensure that only eligible participants are enrolled in the study, saving time and resources by reducing the need for manual screening.
Consent Management
Consent management is a crucial aspect of eRegistration. The system allows participants to electronically provide informed consent, typically through digital signatures or electronic consent forms.
Our eRegistration module includes interactive elements, such as multimedia content or questionnaires, to enhance participant understanding and engagement during the consent process.
The system securely stores and tracks consent documentation, ensuring compliance with ethical and regulatory requirements.
Consent management in eRegistration enhances participant convenience, streamlines the consent process, and improves documentation and accountability.
Usecases
Streamline consent with eRegistration
Discover how our eRegistration consent module optimises clinical trials and academic research, enhancing data collection and participant comprehension.
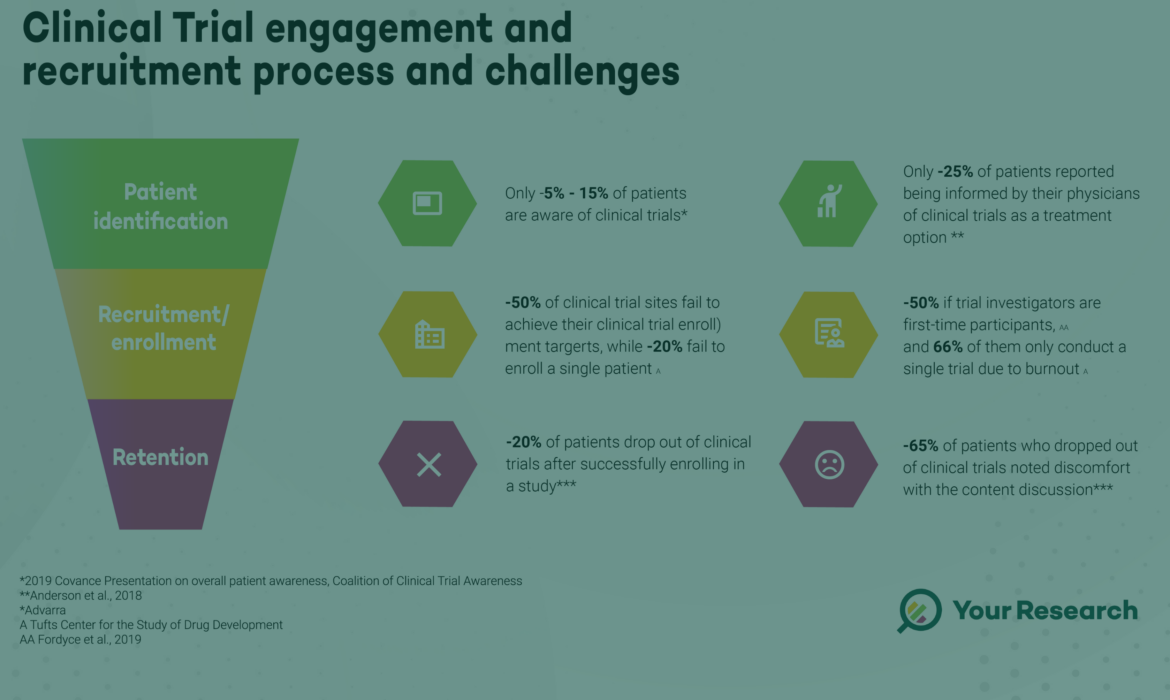
Patient Identification, Recruitment, Enrolment and Retention
Technical specifications
Easy to use, secure & integrable
User-Friendly
Secure
Integrable
- Intuitive and easy-to-navigate interface
- Clear instructions and logical workflows
- Compatibility across devices and screen sizes
- Robust security measures (encryption, secure transmission protocols)
- User authentication mechanisms and access controls
- Compliance with data privacy regulations and standards
- Seamless integration with EDC, EHR, or other systems
- Reduced duplicate data entry
- Real-time data exchange and compatibility with data standards
Related modules
Along the participant journey
With Your Research, you have the flexibility to utilize stand-alone modules, integrate them with your existing software vendors, or connect them to other modules within Your Research to enhance the participant journey.
Enhance your research
Contact us and we will be happy to show you how Your Research makes a difference in conducting clinical research, making research more efficient and personalised for everyone involved.