Best in-class technology; unparalleled service
eCOA excellence
Electronic Clinical Outcome Assessment (eCOA) refers to the use of electronic devices, such as smartphones, tablets, or computers, to collect patient-reported outcome measures, clinical assessments, and other relevant data directly from study participants.
Avoid delays, deliver high-quality data, and retain participants with Your Research eCOA.
* * * * *
4,5/5 user reviews
Read More
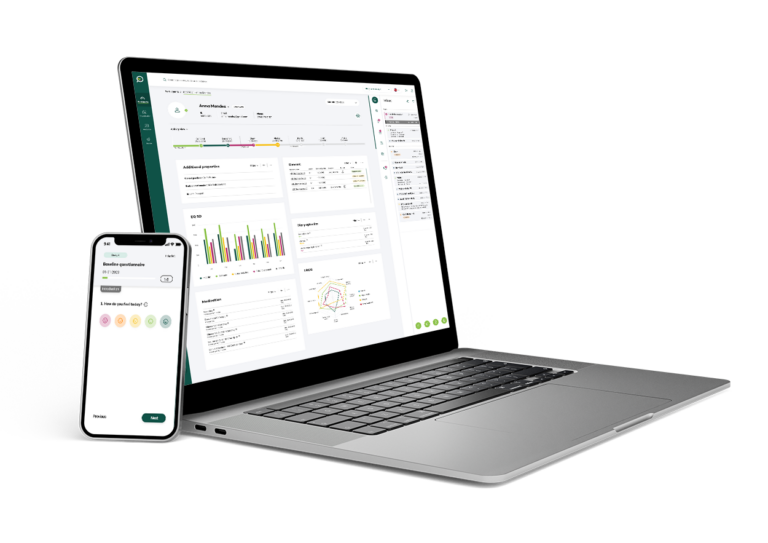
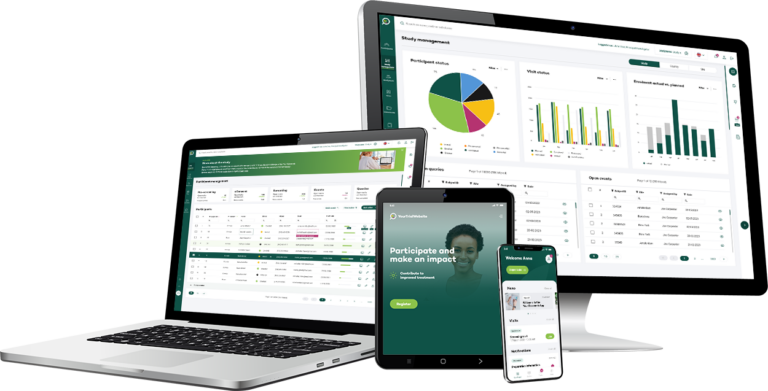
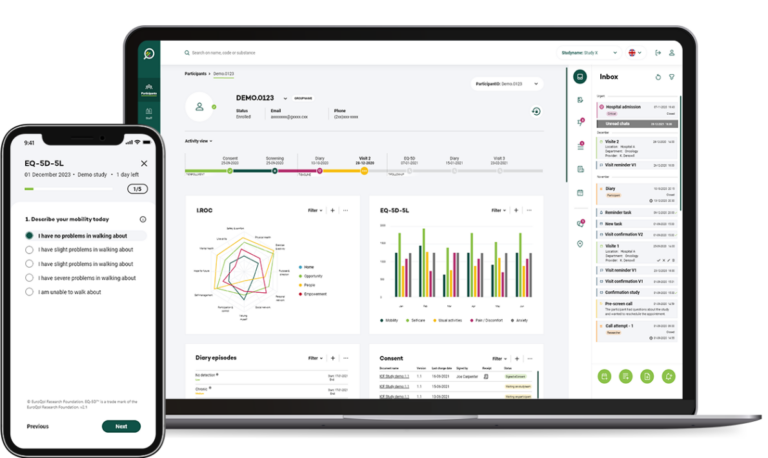
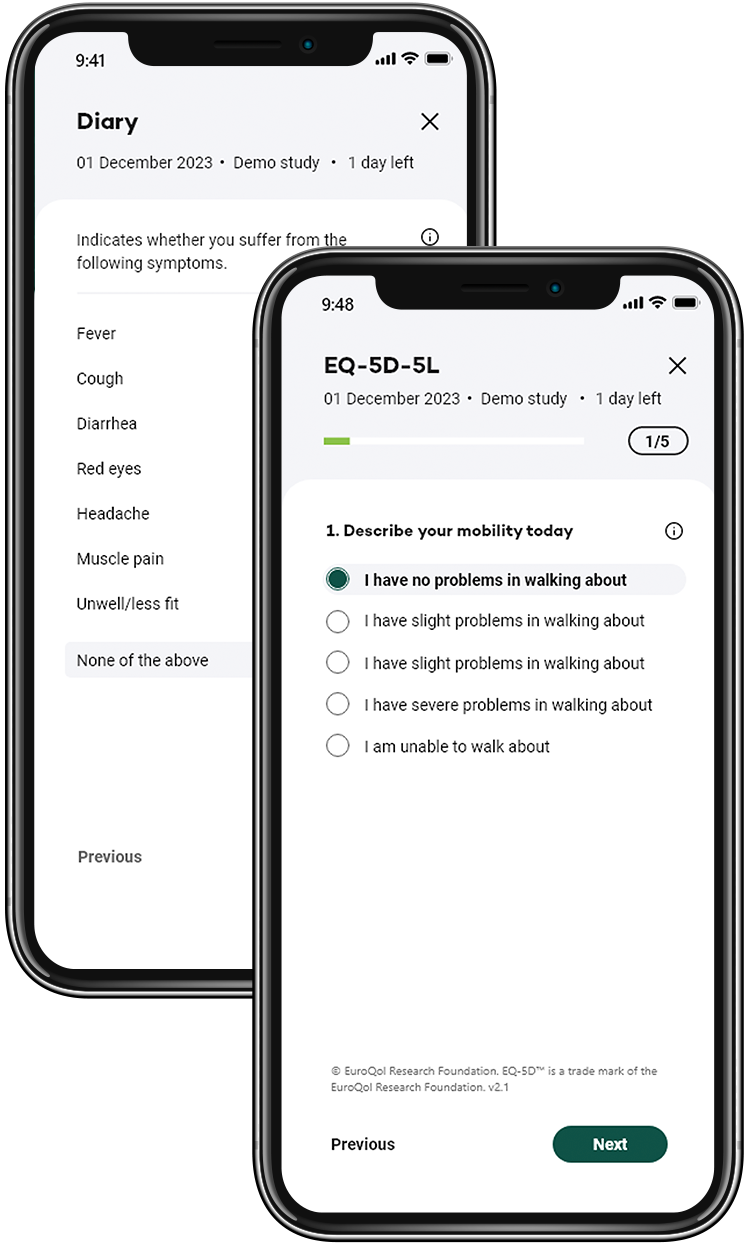
Key features
Enhance the entire patient journey for all stakeholders
We distinguish ourselves by supporting everyone involved in the trial to look ahead, intelligently guiding daily operations towards trial optimisation, with a focus on participant retention and the collection of high-quality data throughout the participant journey.
User-Centric design
Intuitive interfaces and remote data capture capabilities increase patient engagement and compliance.
Real-Time Data Insights
Your Research instantly captures and provides access to data, facilitating timely decision-making and proactive patient management.
Enhanced Data Integrity
Reduce potential data entry errors and ensure high data quality through electronic validations.
Your Research helps maintain data quality and enhances the reliability of clinical trial results.
Global compliance
Your Research complies with international standards and regulations, ensuring data security and privacy across all operational regions.





From self to full service
Streamline service delivery to minimize risks and expedite startup
Support everyone involved in the trial to look ahead; intelligently guide daily operations towards trial optimisation; focus on participant retention and the collection of high-quality data throughout the participant journey; all this with Your Research eCOA.
Validated questionnaires
Use our pre-validated questionnaire library and existing relations with license holders
Translations
Our integrated network of translators will support with translation of questionnaires and communication information with participants.
IRB submission support
Together with our technology and pre-defined documentation, we support you to have visibility and the IRB approval process per country.
Device provisioning
Your Research provides pre-configured devices to patients for consistent performance and controlled study environments.
Training and support
Around-the-clock assistance to address technical issues and user inquiries. Training for clinical staff to ensure they are proficient in using Your Research and guide patients.
Beyond eCOA
Elevate the whole patient journey by adding modules
Fully integrated modules that make a difference and will have a direct positive impact on trial outcomes.
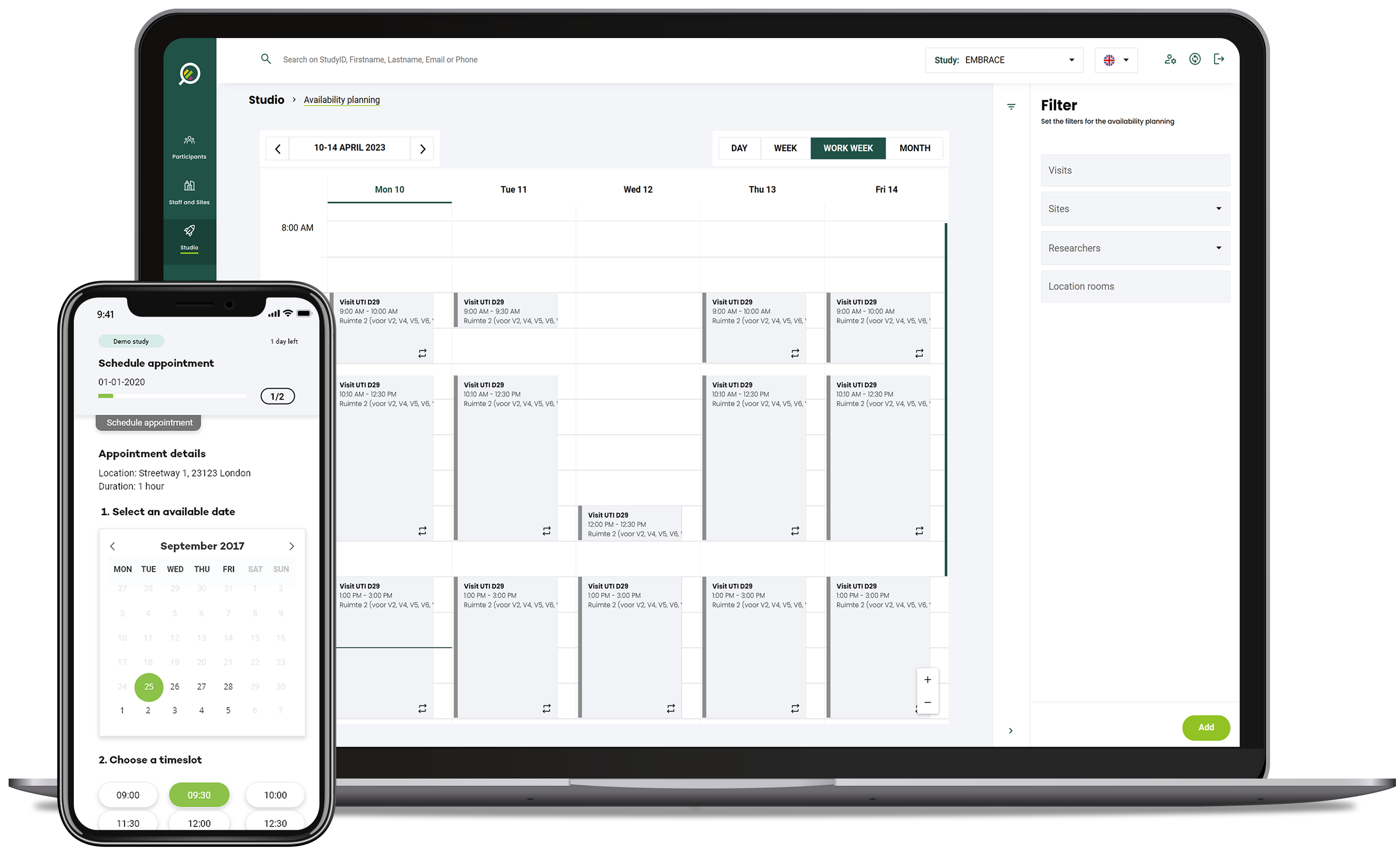
Visit scheduling
Real-time insights into protocol adherence and assistance in scheduling visits according to protocol, while reducing no-shows through proactive participant notifications.
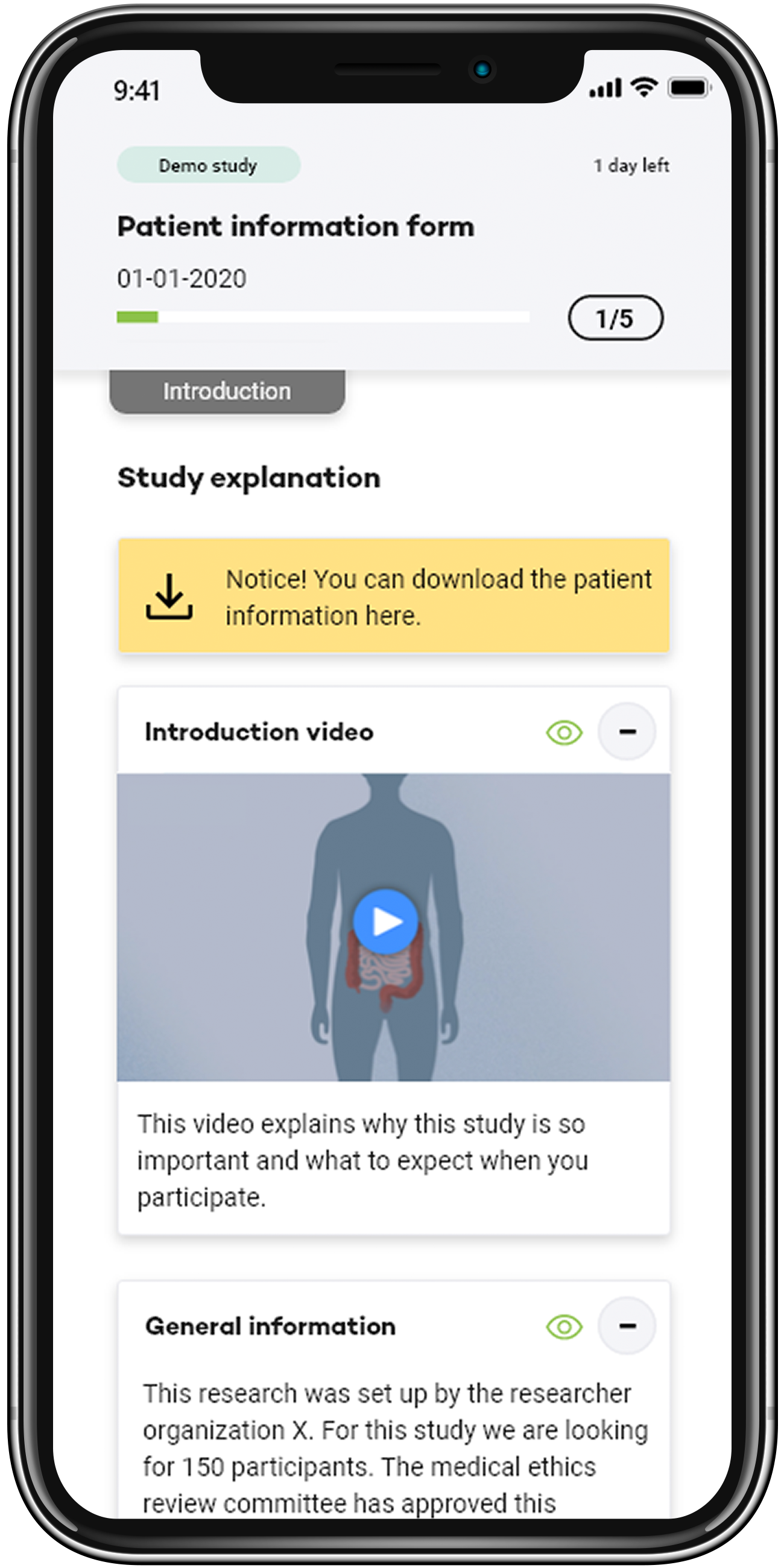
eConsent
eConsent streamlines the informed consent process through digital means, enhancing understanding and facilitating remote participation with interactive, easily accessible information.
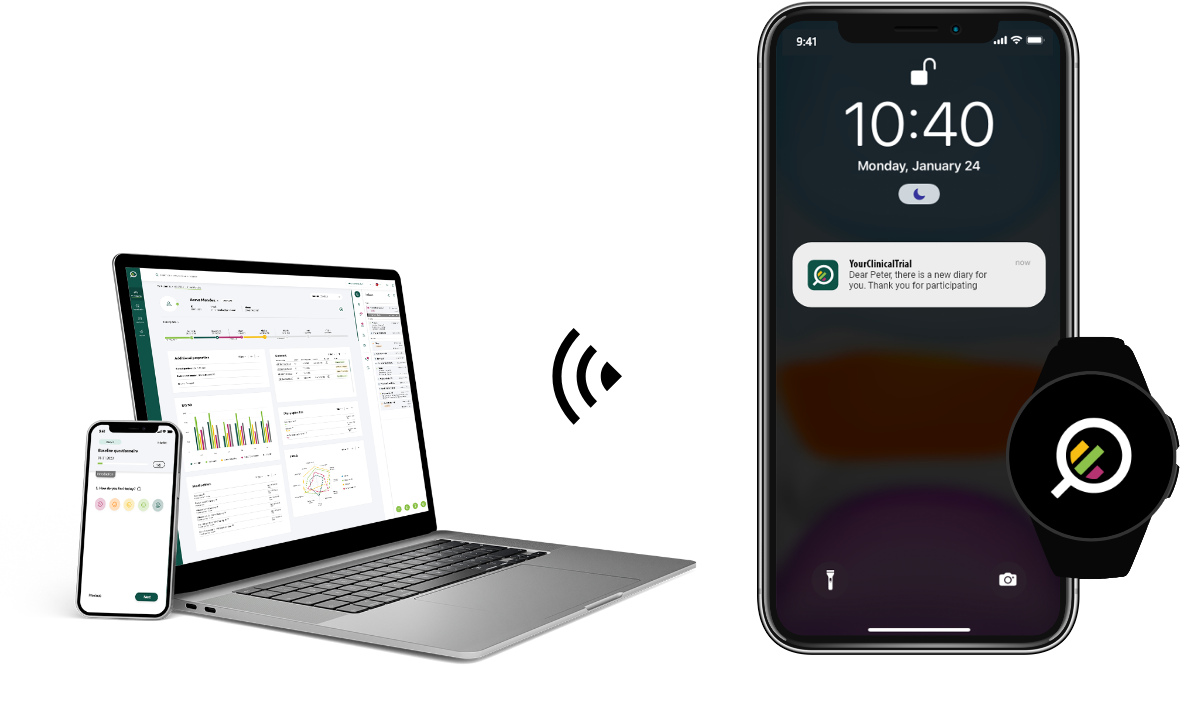
Connected devices
Collect vital values directly through devices such as blood pressure monitors, scales, SpO2 sensors, and activity trackers in Your Research, for real-time monitoring and improvement of patient outcomes.
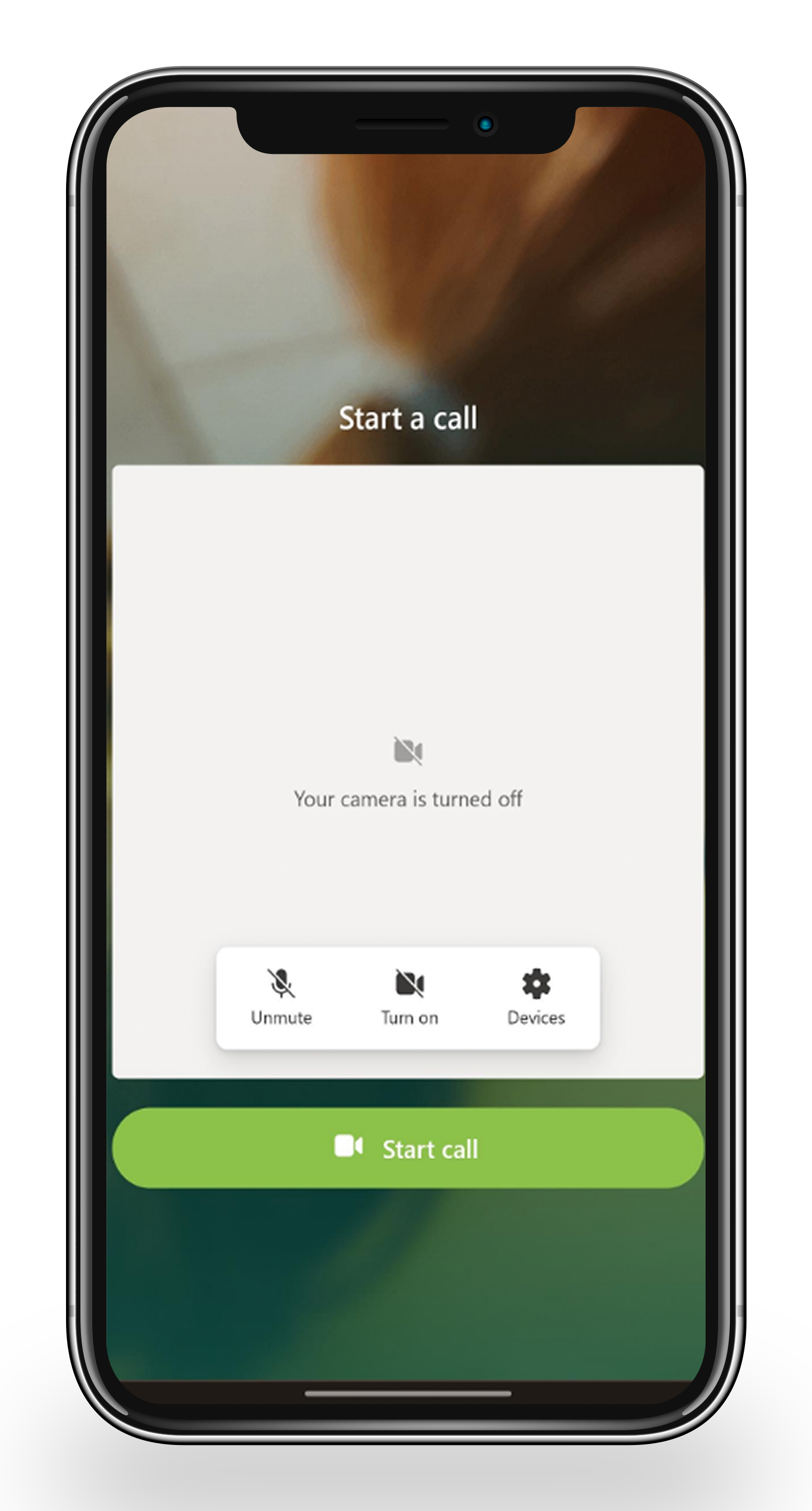
Televisits
Reduce participant burden by enabling remote visits through video calling or homecare nurse support, allowing interactions from home without the need for travel.
Experience efficient trials
Contact us and we will be happy to show you how Your Research makes a difference in conducting clinical research, making research more efficient and personalised for everyone involved.
