Engage participants with outstanding usability
ePRO
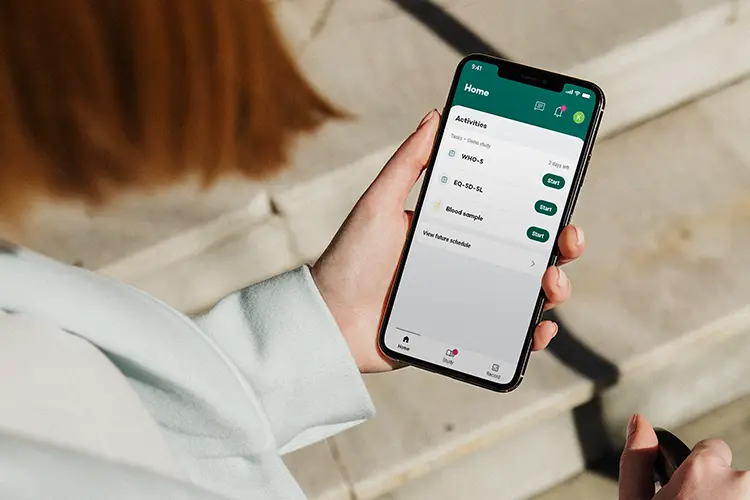
Your Research’s ePRO software is designed to make patient-reported outcomes simple and efficient, enhancing data integrity and compliance throughout the clinical trial process. Explore the key features that set our ePRO solution apart.
Ensures participants can easily navigate and report their outcomes, improving engagement and data accuracy.
Leverages reliable, pre-validated tools to streamline the data collection process.
Personalized notifications and smart data collection deliver real-time insights, boosting participant retention and identifying potential risks.
Key features
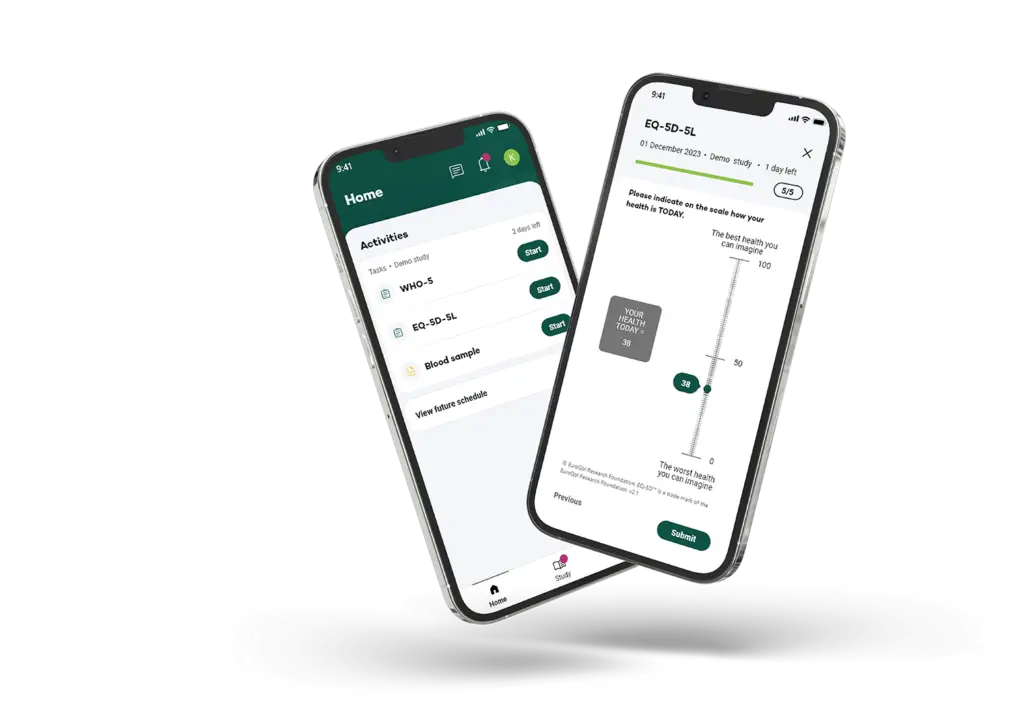
Retain participants and collect high quality data
Leveraging both retrospective and prospective insights, our intelligent workflow guidance supports efficient and accurate trial management.
By providing an exceptional experience, timely notifications, and enhanced usability for participants, we effectively mitigate both study protocol adherence and patient retention risks.
Intuitive design for ease of use by patients of all ages.
Multilingual support to cater to diverse patient populations.
Instant access to patient data for timely insights.
Seamless integration with all types of devices
Tailor Forms to meet the specific needs of your study.
Utilise a variety of question formats, including multiple-choice, Likert scales, and free-text responses.
Automated reminders to enhance patient adherence.
Interactive features to keep patients engaged and motivated.
Advanced analytics to monitor and analyse patient data.
Customisable reports for easy sharing with stakeholders.
Other Types of Assessments
Your Research facilitates efficient data management, organisation, and analysis, enabling study teams to smartly and proactively access and analyse patient-reported data more easily.
Our services
Streamline service delivery to minimize risks and expedite startup
Minimising startup and delay risks is the goal of our services. Whether you prefer a hybrid or full self-service model, we are ready to assist with our expertise and experience, ensuring peace of mind.
Pre-validated questionnaires will save you time setting up your trial and data quality risks
Our network of translators will support with validated translation of questionnaires and communication information for participants.
Together with our technology, local partners and pre-defined documentation, we support you to have oversight into your IRB approval process.
Your Research provides pre-configured devices to patients for consistent performance and controlled study environments based on a hybrid or full provisioning model.
We provide 24/7 assistance to address technical issues and user inquiries, as well as training for clinical staff to ensure they are proficient in using Your Research.
Modules at glance
Elevate the whole participant journey
Serving as the essential foundation, our unified protocol enhances trial outcomes and facilitates the seamless integration of both modules and third-party systems along the participant journey.
This foundational structure ensures comprehensive oversight and enables strategic foresight throughout the patient journey.





