Your Research supports
Innovative CROs who choose to embrace technology
In the rapidly evolving landscape of the clinical trial industry, digital software solutions have emerged as the driving force behind transformative changes.
Contract Research Organisations (CROs) are at the forefront of adopting these innovations to enhance efficiency, accuracy, and overall research quality.
This page explores the vital role that digital software solutions play in the clinical trial industry, focusing on the benefits they bring to CROs and the specific areas where digital innovation is poised to revolutionise research.
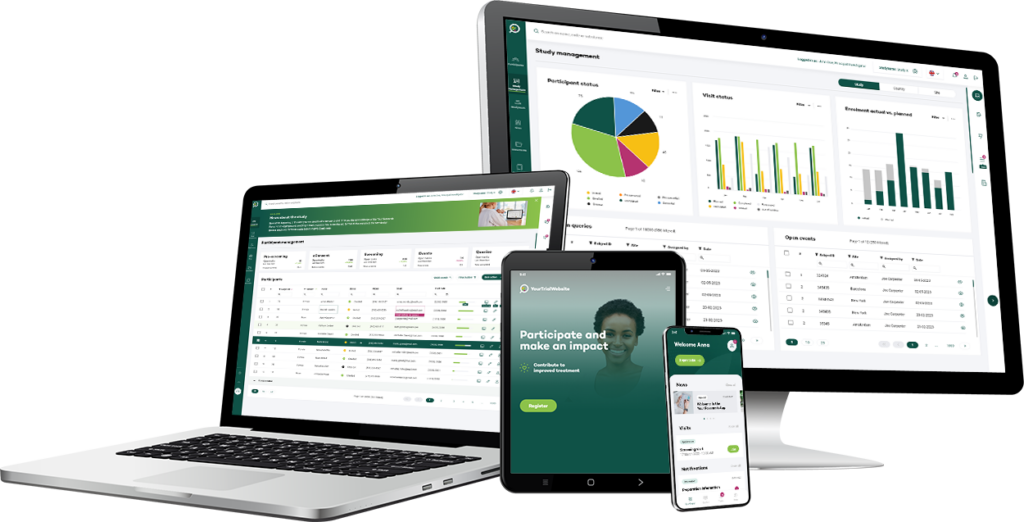
Key features
Innovation starts with you
The clinical trial industry stands on the precipice of a digital revolution, and Contract Research Organisations are harnessing the power of digital software solutions to drive progress.
Enhanced efficiency, improved data quality, and adherence to regulatory standards are just a few of the benefits that CROs derive from these solutions.
Artificial Intelligence (AI) and Machine Learning (ML)
AI and ML technologies hold immense promise in optimising clinical trials.
Predictive analytics can help CROs identify suitable investigational sites, predict patient recruitment challenges, and optimise resource allocation.
These technologies can also enhance data analysis, aiding in the identification of subtle patterns and correlations within complex datasets.
AI-powered algorithms contribute to more informed decision-making, driving efficiency and reducing costs.
Real-time Data Analytics and Insights
The traditional approach of collecting data, followed by delayed analysis, is being replaced by real-time data analytics.
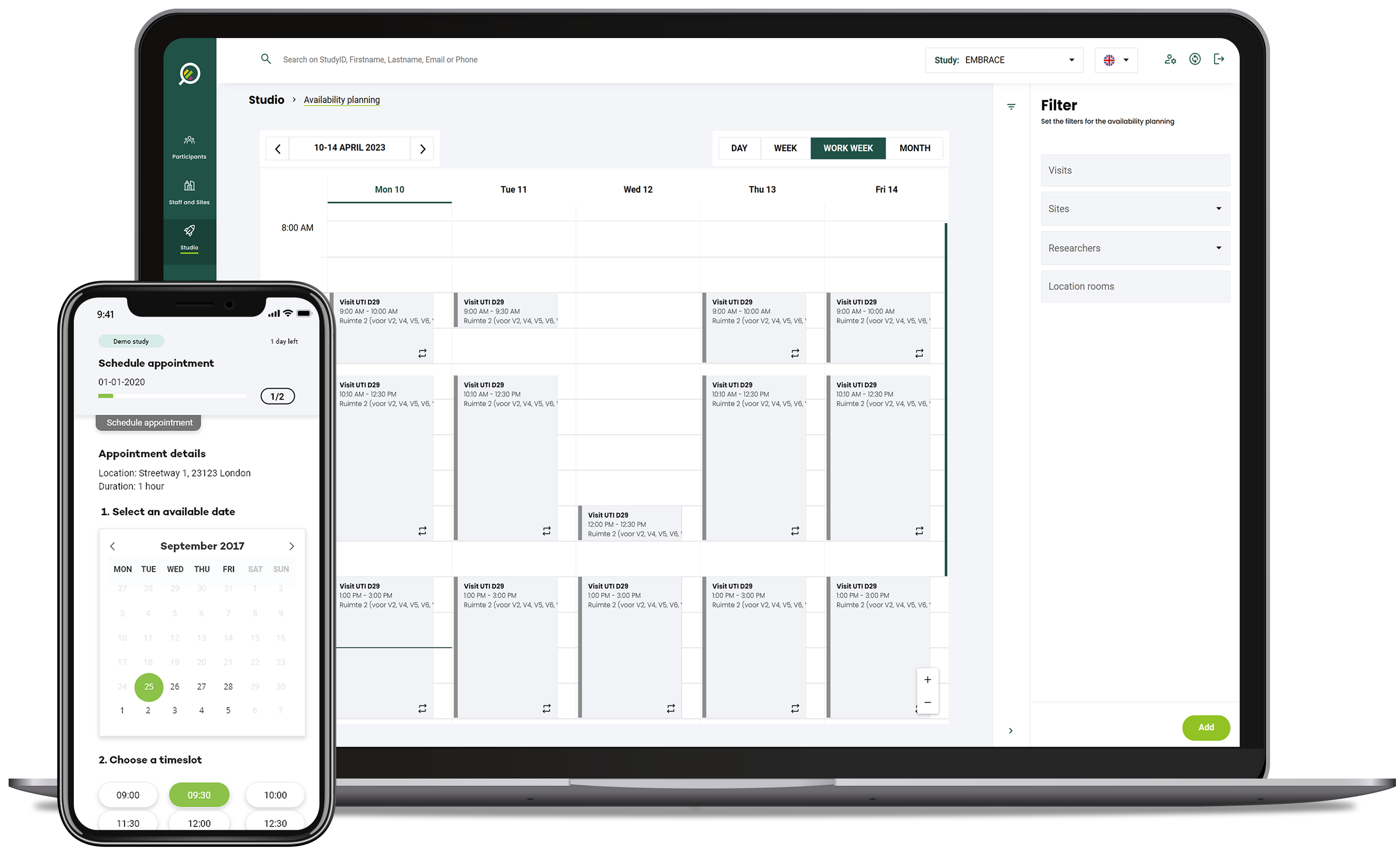
Your Research's Unified Protocol approach allows CROs to monitor trial progress and analyze data as it's generated.
This real-time insight enables quicker identification of trends, potential safety concerns, and efficacy signals.
Adaptive trial designs driven by instant data analysis contribute to more agile decision-making and improved trial outcomes.
Patient Engagement and Recruitment
Digital innovation is poised to revolutionise patient recruitment and engagement strategies.
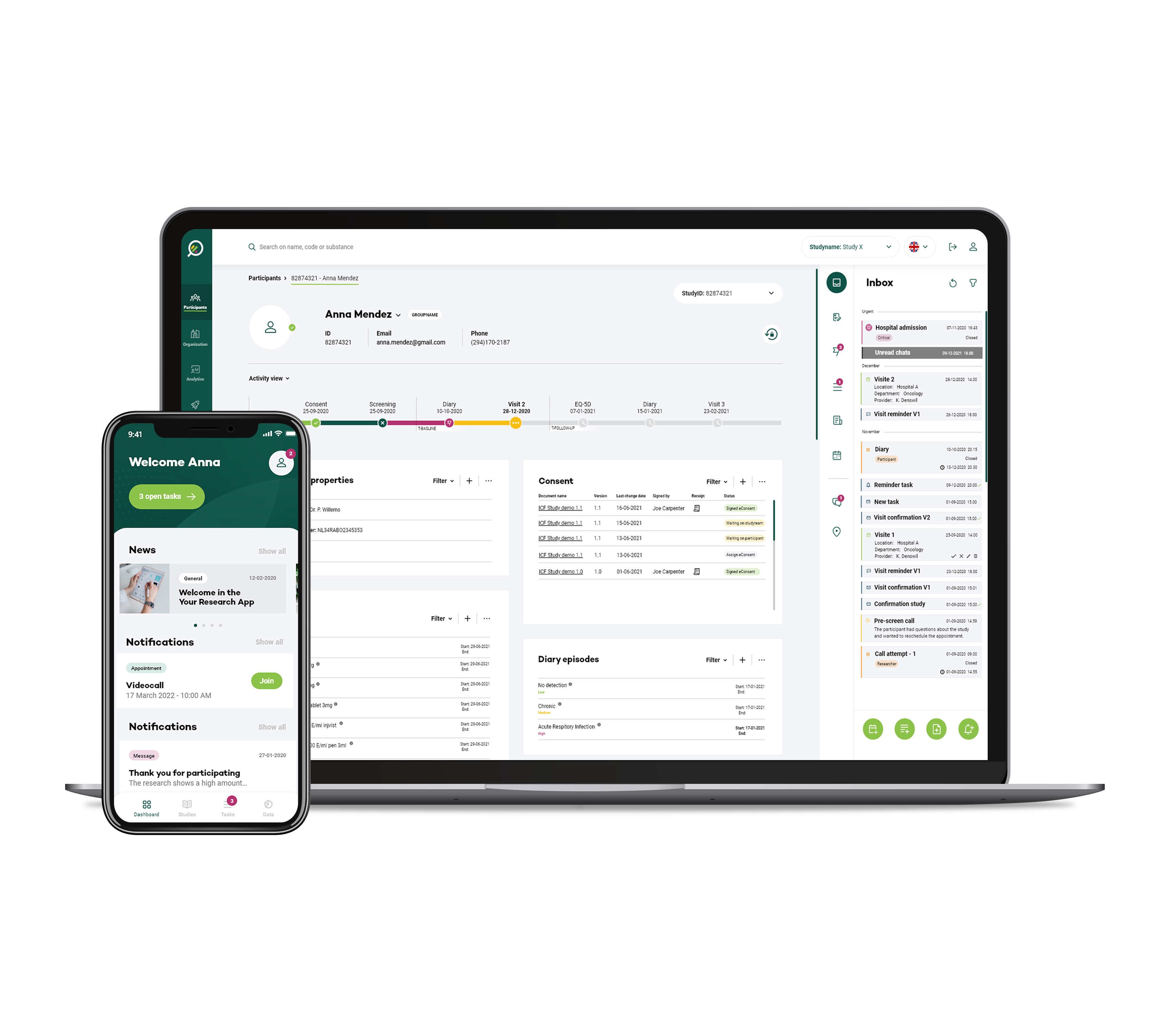
Our mobile app, wearable devices integration, and online platform can connect patients with clinical trials that match their profiles and conditions.
These solutions enhance communication between researchers and participants, promote trial awareness, and facilitate real-time data collection, ultimately leading to higher recruitment rates and increased patient retention.
Key benefits
Enhance your oversight
Enhanced Efficiency and Speed
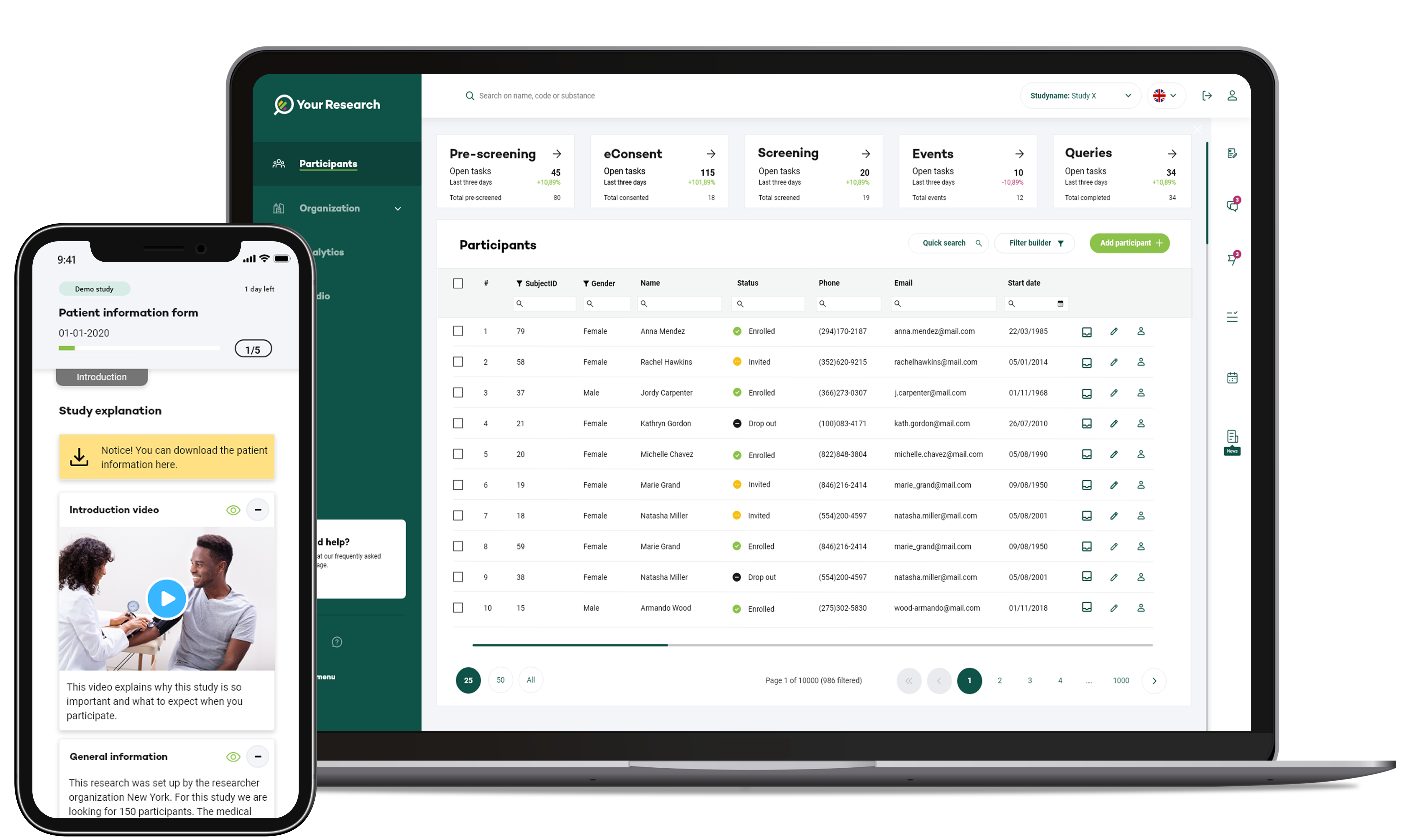
Our digital software solutions streamline every facet of the clinical trial process, from protocol design to data collection and analysis.
These solutions enable CROs to manage complex tasks with unprecedented efficiency, reducing the time it takes to initiate, conduct, and complete trials.
Automated workflows and real-time collaboration tools expedite decision-making and ensure that trials progress smoothly.
Data Accuracy and Quality
Accurate and reliable data are paramount in clinical trials. Digital software solutions ensure data integrity through advanced validation and verification mechanisms. Electronic data capture (EDC) systems eliminate the risks associated with manual data entry, minimizing errors and ensuring compliance with rigorous regulatory standards. High-quality data not only accelerate the trial process but also contribute to more meaningful and conclusive results.
Compliance and Regulatory Adherence
Navigating the complex landscape of regulatory compliance is a cornerstone of clinical research. Digital software solutions provide CROs with tools to maintain meticulous documentation, adhere to industry regulations, and ensure the highest standards of ethical conduct. Automated tracking of regulatory changes, integration of compliance protocols, and secure data storage enable CROs to operate seamlessly within the bounds of regulatory frameworks.
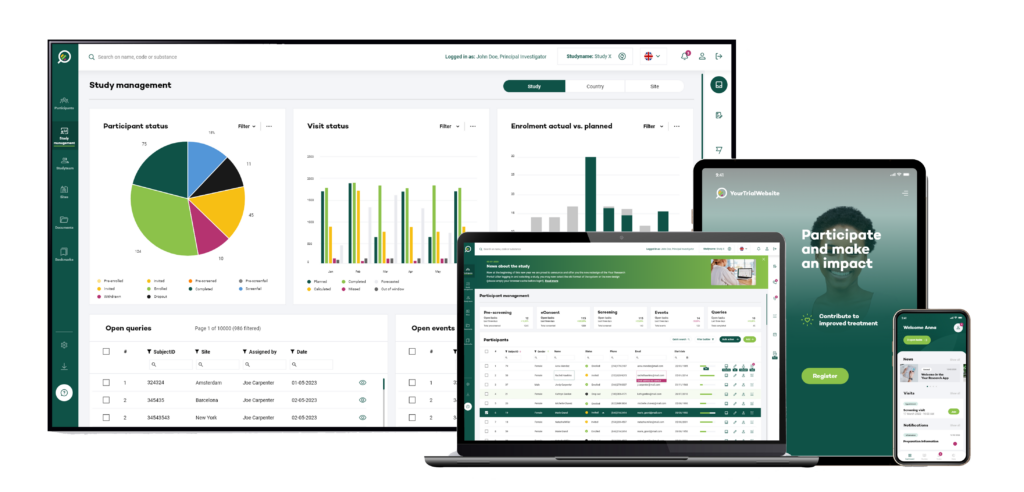
Your Research bespoke solutions for CROs
Interchangeable modules streamlining operations
With Your Research, you can utilise stand-alone modules,
integrate them with your existing software vendors, or connect them to other modules within Your Research to enhance the participant journey.
Click on one of the solutions to go directly to the solution page for more information.
Further benefits for
Patient Enrolment Rates
Patient enrolment is a pivotal phase of clinical trials, directly impacting the success and efficiency of the entire research process.
In recent years, the integration of digital technology has revolutionised patient recruitment for Contract Research Organisations (CROs), offering a range of benefits that accelerate enrolment rates and enhance the overall trial experience.
Here’s how digital technology is transforming patient recruitment for CROs:
Wider Reach and Targeted Outreach
Our digital technology enables CROs to reach a broader and more diverse audience by leveraging various online platforms and tools.
Social media, search engine optimisation (SEO), and targeted online advertising allow CROs to precisely identify potential participants who match the trial's criteria.
This targeted approach not only increases the pool of eligible candidates but also ensures that recruitment efforts are focused on individuals who are more likely to be interested and qualified.
Enhanced Patient Engagement
Engaging potential participants and maintaining their interest throughout the trial process is crucial for enrolment success. Our digital tools, such as mobile apps, websites, and online communities, provide CROs with platforms to interact with patients directly. These platforms offer detailed trial information, enable online pre-screening, and facilitate two-way communication. Patients can ask questions, clarify doubts, and receive real-time updates, fostering a sense of involvement and trust. This heightened engagement significantly reduces drop-out rates and encourages participants to remain committed to the trial.
Convenience and Accessibility
Our digital technology eliminates geographical barriers and offers a level of convenience that traditional methods cannot match. Virtual consultations, tele-health appointments, and remote monitoring enable patients to participate in trials from the comfort of their homes. This convenience is especially advantageous for individuals with mobility issues, those residing in remote areas, or those who have demanding schedules. By making participation more accessible, CROs can tap into a larger and more diverse participant pool, accelerating enrolment timelines.
Data-Driven Insights
Our digital platform collects valuable data on participant behaviours, interactions, and preferences. CROs can analyse this data to refine their recruitment strategies continuously. By understanding which platforms yield the best engagement, which information resonates most with participants, and how potential participants are navigating through the recruitment process, CROs can tailor their approaches for maximum impact. This data-driven approach optimises recruitment efforts and ensures that resources are allocated to the most effective channels.
Real-Time Feedback and Adaptation
Your Research digital technology allows CROs to receive real-time feedback from potential participants. This feedback loop enables CROs to make swift adjustments to their recruitment materials, processes, and messaging. If certain aspects of the trial design or recruitment process are dissuading potential participants, CROs can identify and rectify these issues promptly. By addressing concerns in real-time, CROs can maintain a positive perception of the trial and increase the likelihood of successful enrolment.
Competitive Edge
Stay ahead of competitors by leveraging innovative technology to conduct more efficient and patient-centric trials, attracting sponsors and maintaining a reputation for excellence.
Further benefits for
Empowering Risk Mitigation
Effective risk mitigation is a cornerstone of successful clinical trials for Contract Research Organisations (CROs). In the ever-evolving landscape of clinical research, digital technology has emerged as a powerful ally, offering a range of benefits that enhance risk identification, assessment, and management.
Here’s how digital technology is revolutionising risk mitigation for CROs in clinical trials:
Early Detection and Proactive Risk Identification
AI and ML technologies hold immense promise in optimising clinical trials.
Predictive analytics can help CROs identify suitable investigational sites, predict patient recruitment challenges, and optimise resource allocation.
Your Research's digital tools enable CROs to capture and analyse vast amounts of data in real-time. This data-driven approach allows for the early detection of potential risks and anomalies, which might have gone unnoticed through traditional methods. By continuously monitoring various parameters such as patient safety data, adverse events, and protocol adherence, CROs can identify potential risks at their nascent stages, enabling timely intervention and mitigation strategies.
Enhanced Data Analytics and Predictive Modeling
Our digital technology offers advanced data analytics capabilities that empower CROs to identify trends, patterns, and correlations within complex datasets. By harnessing the power of machine learning and predictive modelling, CROs can anticipate potential risks based on historical data and make informed decisions to prevent their occurrence. This proactive approach allows CROs to allocate resources strategically, optimise trial designs, and implement risk-reducing interventions before issues escalate.
Centralised and Real-time Communication
Our digital platforms facilitate seamless communication and collaboration among the various stakeholders involved in clinical trials. From investigators and sponsors to regulatory bodies and participants, all can stay connected in real-time. This centralisation of communication ensures that any emerging risks or challenges are communicated swiftly and accurately to all relevant parties. Rapid dissemination of information enables stakeholders to respond promptly and collaboratively, minimising the impact of potential risks.
Remote Monitoring and Adverse Event Reporting
Our digital technology enables remote monitoring of participants, reducing the need for frequent in-person visits. Wearable devices, mobile apps, and electronic patient diaries can collect real-time data on patient health and experiences. This constant monitoring allows for the early detection of adverse events, enabling CROs to intervene promptly. Additionally, participants can report adverse events directly through our digital platform, enhancing reporting accuracy and reducing delays in addressing safety concerns.
Compliance and Regulatory Adherence
Maintaining compliance with regulatory standards is a paramount concern for CROs. Your Research's digital technology provides tools for accurate documentation, secure data storage, and automated reporting, ensuring that CROs meet regulatory requirements. By adopting digital solutions that facilitate proper documentation and adherence to industry standards, CROs mitigate the risk of non-compliance, which could otherwise lead to delays, fines, or trial suspension.
Scenario Planning and Simulations
Our digital technology allows CROs to simulate various scenarios and assess their potential impact on the trial. This aids in proactive risk assessment and mitigation strategy development. By simulating different protocol adjustments, patient recruitment rates, or unforeseen events, CROs can make informed decisions that minimise risk exposure and ensure smooth trial progression.
Further benefits for
Decreasing Protocol Deviations
In the dynamic landscape of clinical trials, protocol deviations are inevitable but managing them efficiently is paramount to ensure trial integrity, patient safety, and regulatory compliance.
Contract Research Organisations (CROs) play a pivotal role in this process, and digital technology has emerged as a game-changer in the management of protocol deviations.
Here are the benefits that digital technology brings to CROs in handling protocol deviations for clinical trials:
Real-time Detection and Reporting
Your Research's digital solutions enable CROs to detect and report protocol deviations in real-time. With electronic data capture (EDC) systems, data entered by sites are automatically subjected to validation rules, helping identify discrepancies immediately. This instant detection allows CROs to address deviations promptly and take corrective actions sooner, reducing the potential impact on trial outcomes and data integrity.
Structured Documentation and Tracking
Our digital platform offers a structured and standardised way to document and track protocol deviations. CROs can capture deviations along with relevant details such as their causes, impacts, and corrective measures taken. This organised documentation streamlines communication among various stakeholders, ensuring that all parties are informed and aligned. Additionally, the tracking feature helps in monitoring trends and identifying recurring issues for proactive prevention.
Automated Workflow and Escalation
Your Research's digital technology enables CROs to implement automated workflows for managing protocol deviations. When a deviation occurs, the system can trigger predefined workflows that guide the responsible parties through the necessary steps for assessment, documentation, and resolution. This streamlines the process, reduces manual intervention, and ensures that deviations are handled consistently and efficiently across different trials.
Data Analysis for Root Cause Identification
Our digital modules facilitate comprehensive data analysis, aiding in identifying the root causes of protocol deviations. By aggregating data from multiple sources, CROs can analyse trends and patterns that contribute to deviations. This data-driven approach enables CROs to address underlying issues in trial processes or site practices, leading to targeted interventions that reduce the likelihood of future deviations.
Remote Monitoring and Real-time Auditing
Our digital modules allows for remote monitoring of trial sites and data, making it easier to identify protocol deviations without the need for frequent on-site visits. This capability is especially beneficial for global trials or sites located in remote areas. Real-time auditing features further enhance the ability to identify deviations, providing auditors with access to the most up-to-date information for assessment.
Regulatory Compliance and Reporting
Your research's digital platforms assist CROs in ensuring regulatory compliance when handling protocol deviations. The structured documentation, automated workflows, and standardised reporting formats align with regulatory requirements, making it easier to compile and submit deviation-related information to regulatory authorities. This reduces the risk of compliance issues and delays in trial progression.
Further benefits for
Elevating Clinical Trial Monitoring
Monitoring visits are a cornerstone of ensuring the integrity, compliance, and quality of clinical trials conducted by Contract Research Organisations (CROs). The integration of digital technology has brought forth transformative benefits that enhance the effectiveness and efficiency of monitoring visits.
Here’s how digital technology is revolutionising monitoring visits for CROs in clinical trials:
Real-time Data Access and Review
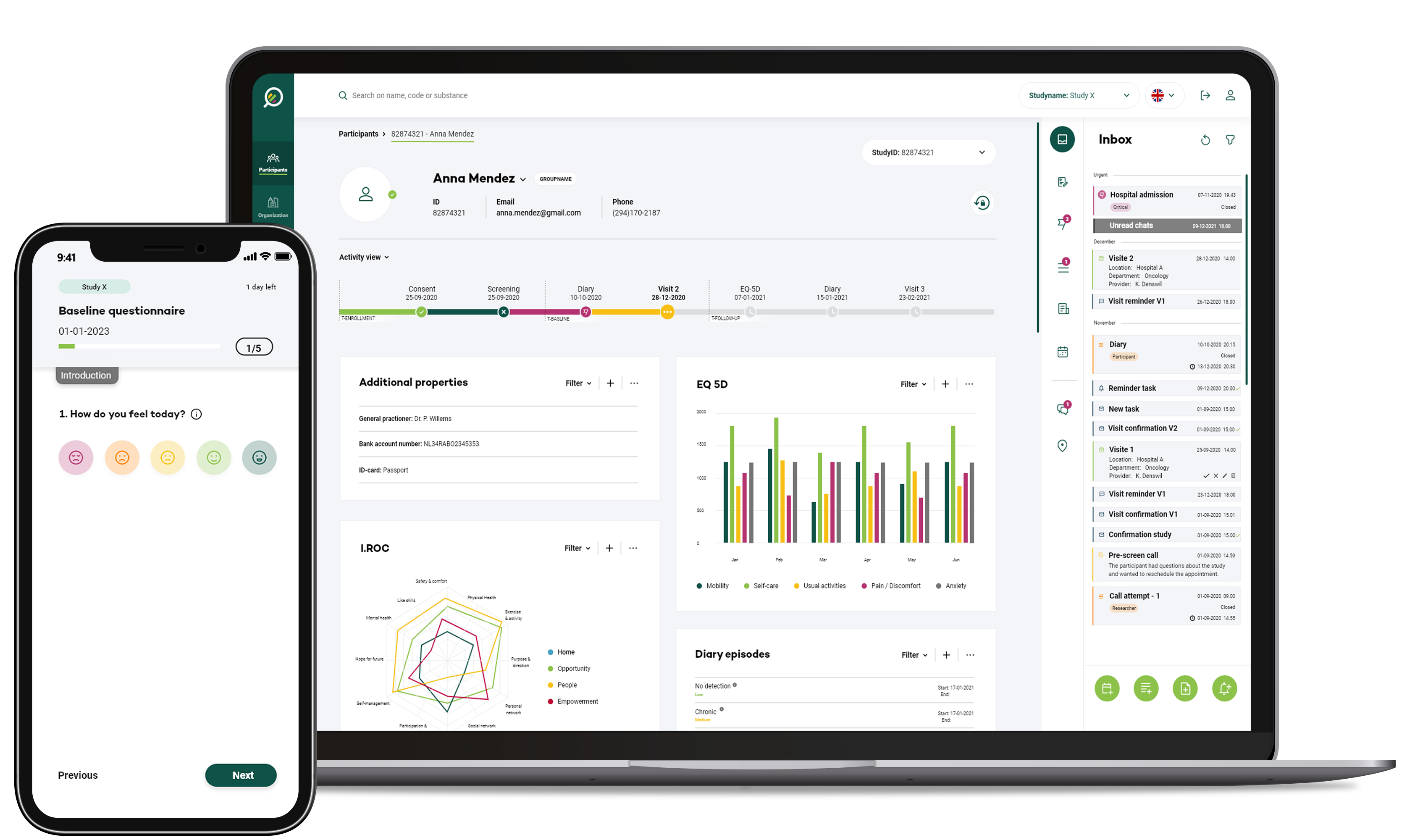
Your Research's digital technology enables CROs to access trial data and site activities in real time, linking with Electronic Data Capture (EDC) systems and cloud-based platforms allow for immediate access to patient data, case report forms, and trial documentation. This real-time access empowers monitors to review data promptly, identify trends, and detect potential issues as they emerge, reducing the time lag between data collection and review.
Remote Monitoring and Virtual Visits
Our digital platform facilitate remote monitoring capabilities, reducing the need for physical visits to trial sites. Virtual visits, video conferencing, and remote data access enable monitors to assess site activities without the constraints of travel. This approach improves the efficiency of monitoring, reduces costs, and enables more frequent interactions between monitors and site staff.
Data Quality Oversight
Your Research's modules offer sophisticated data quality oversight mechanisms. Monitors can use electronic platforms to identify inconsistencies, missing data, and errors in real-time. Automated alerts and validation checks notify monitors of potential data issues, allowing for prompt corrective action. This ensures the integrity of trial data and enhances its accuracy for analysis and reporting.
Risk-based Monitoring
Our digital modules support risk-based monitoring strategies. By analysing data trends and patterns, CROs can focus monitoring efforts on high-risk areas or sites, optimising resource allocation. Our online platforms provide the necessary tools to implement risk-based monitoring plans effectively, ensuring that the most critical aspects of the trial are closely monitored.
Streamlined Communication
Your Research's modular platform enables seamless communication between monitors, investigators, and site staff. Monitors can provide feedback, ask questions, and clarify concerns through online collaboration tools. This real-time communication enhances the exchange of information and facilitates quick resolution of queries or issues that arise during monitoring visits.
Auditing and Compliance Tracking
Our digital technology simplifies the tracking of audit trails and compliance-related activities. Monitors can document their observations and actions directly into digital platforms, leaving an electronic record of the visit. This transparent documentation streamlines the auditing process, making it easier to track and verify compliance with regulatory standards.
Seamless Integration for Enhanced Efficiency
Eliminating Administrative Burdens and Protocol Deviations
Your Research recognises the significance of incorporating your existing research systems to enhance the efficiency of your operations.
Thus we provide API (Application Programming Interface) and SSO (Single Sign On) software to integrate your existing systems, such as Electronic Data Capture (EDC), Clinical Trial Management Systems (CTMS), and Electronic Health Records (EHR).
Connect other modules and existing systems
Enhance your efforts with Your Research
These streamlining tool helps to reduce administrative burden, accelerate the recruitment timeline, and improve overall efficiency in enrolling eligible participants.
Existing Patient Database
One of the most effective ways to recruit participants is by tapping into an existing patient database.
Your Research has access to a comprehensive and well-maintained database of individuals who have expressed interest in participating in research studies.
By leveraging this resource, we can quickly identify potential candidates who meet your study’s specific criteria.
This approach saves valuable time and resources, allowing you to focus on your research objectives.
Site Networks
Tap into our comprehensive feasibility and site selection services.
We have extensive experience in collaborating with site networks to ensure a seamless identification and engagement process for potential participants.
Through our established connections with local healthcare providers, medical clinics, and community organisations, we can leverage these networks to enhance efficiency and increase the likelihood of finding eligible candidates who are eager to contribute to your research.


Site Networks
Tap into our comprehensive feasibility and site selection services.
We have extensive experience in collaborating with site networks to ensure a seamless identification and engagement process for potential participants.
Through our established connections with local healthcare providers, medical clinics, and community organisations, we can leverage these networks to enhance efficiency and increase the likelihood of finding eligible candidates who are eager to contribute to your research.
Technical specifications
Integrate and customise
Secure Data Management
Scalability and Performance
Integration Capabilities
- We prioritise data security and privacy
- Robust security measures are in place to protect participant information, which adhere to relevant data protection regulations such as GDPR or HIPAA
- Best practice encryption and secure data storage practices ensure the confidentiality and integrity of participant data.
- Serves as a central hub for trial recruitment
- Can handle high volumes of traffic and user interactions.
- Designed to scale effectively, ensuring that it can accommodate increased user activity without compromising performance or causing downtime.
- Integrate with other systems and platforms to enhance functionality and streamline processes.
- Has capability to integrate with databases, electronic data capture (EDC) systems, participant management systems, or electronic health records (EHR) systems.
- This integration enables seamless data exchange, automates data capture, and facilitates efficient participant management throughout the trial lifecycle.
Offering innovative solutions
Your Research is committed to serving CROs
Contact us to see how Your Research makes research more efficient for everyone involved.