Our solution
Solving multiple system fatigue
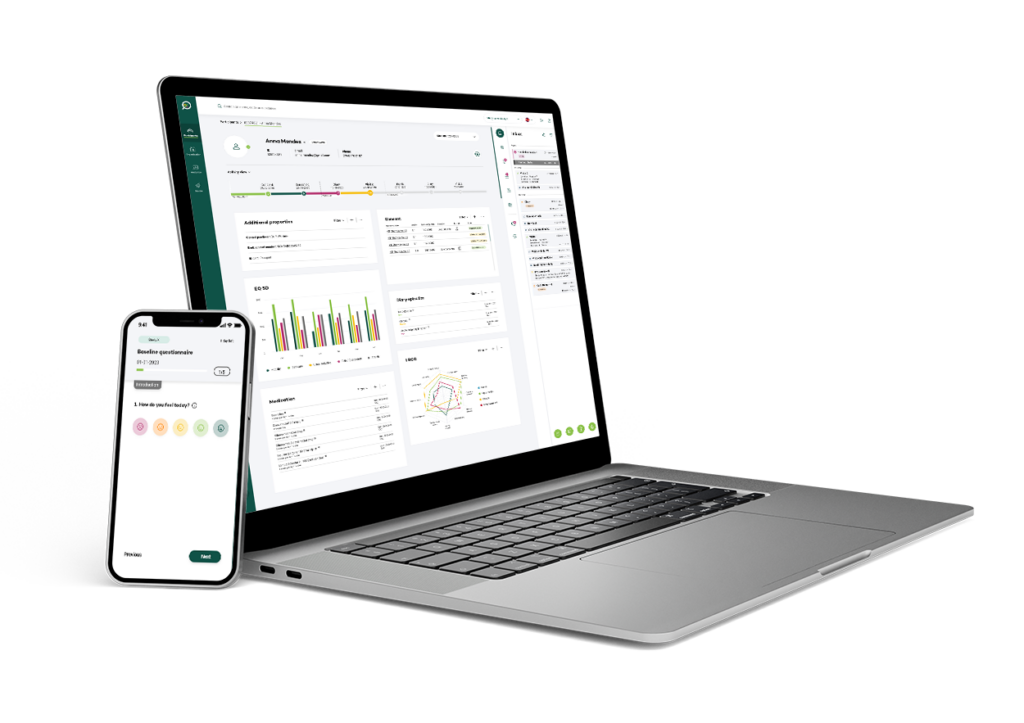
Research advances healthcare, and so should the tools that enable researchers.
We assist Sponsors, CROs and Sites maintain realtime oversight by connecting your existing Data Management systems into one user-friendly interface, as well as provide stand alone focus specific modules so you only use what you need.
Your Research offers turnkey, modular, and bespoke software solutions across the horizontal participant journey, with simple easy-to-use modules that can integrate with other existing systems if required.
Examples of these include:
- EDC systems
- Digital Biomarkers
- Electronic Health Records
- Laboratory Information Management Systems
- Interactive Response Technology Research

Integrate with
Data Management Systems
-
Electronic Data Capture(EDC) Systems
Essential tools for electronic data collection in clinical trials, improving accuracy and speed.
-
Clinical Data Management System (CDMS)
Used to manage and store patient data, allowing for input, validation, cleaning, and management of clinical trial data.
-
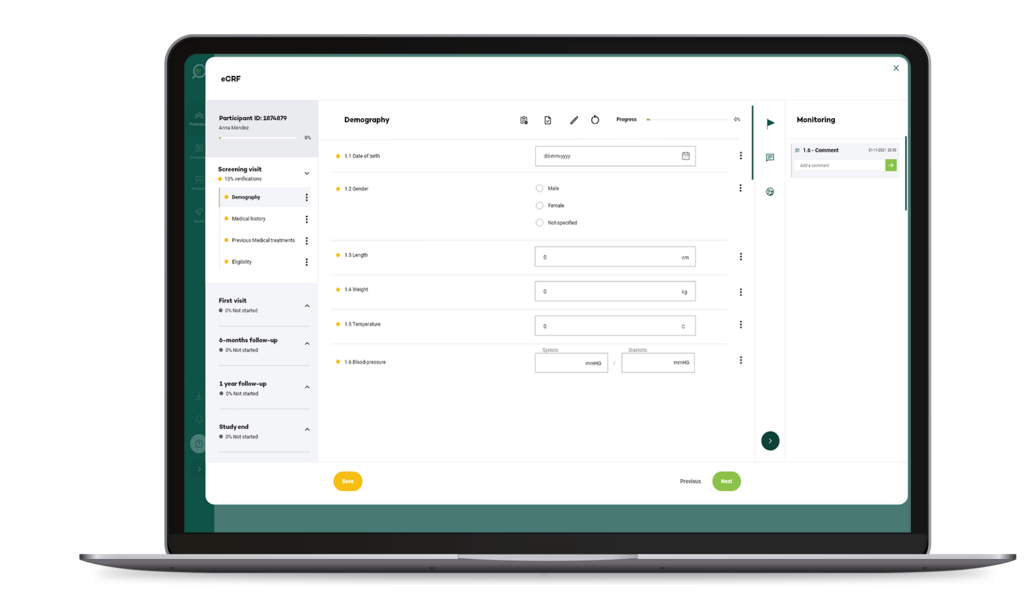
Electronic Case Report Form (eCRF) Systems
Web-based platforms for data collection in clinical trials using pre-designed forms to capture structured participant data for statistical analysis.
-
Randomisation and Trial Supply Management (RTSM) Systems
Also known as Interactive Response Technology (IRT), these systems manage patient randomisation and trial supply, adhering strictly to trial protocols.
-
Clinical Trial Management Systems (CTMS)
These systems manage clinical trials within a clinical research organisation, tracking timelines, participant information, and trial milestones.
-
Electronic Patient-Reported Outcomes (ePRO) Systems
Capture patient-reported outcomes via electronic systems, usually through digital devices. Utilised in clinical trials for collecting information on symptoms, health status, or treatment satisfaction directly from patients.
-
Electronic Clinical Outcome Assessments (eCOA)
Measure a patient’s symptoms, overall mental state, or the effects of a disease in clinical trials. Utilised for accurate and reliable patient-reported, clinician-reported, and performance outcomes.
Integrate with
Medical Devices &
Wearables
-
Wearable Monitoring Devices
Used in clinical trials for continuous patient monitoring. Can track parameters like heart rate, blood pressure, glucose levels, activity levels, and sleep patterns.
-
Imaging Devices
Includes MRI, CT scanners, Ultrasound machines, and PET scanners. Used for diagnosis, monitoring disease progression, and assessing treatment response.
-
Infusion Pumps
Used for precise delivery of medications or nutrients in trials, from chemotherapy drugs to insulin.
-
Spirometers
Devices measuring lung function, used in clinical trials involving respiratory diseases like asthma and COPD.
-
ECG/EKG Machines
Widely used in trials to monitor heart function and detect abnormalities, especially in cardiovascular studies.
Integrate with
Medical Devices &
Wearables
-
Wearable Monitoring Devices
Used in clinical trials for continuous patient monitoring. Can track parameters like heart rate, blood pressure, glucose levels, activity levels, and sleep patterns.
-
Imaging Devices
Includes MRI, CT scanners, Ultrasound machines, and PET scanners. Used for diagnosis, monitoring disease progression, and assessing treatment response.
-
Infusion Pumps
Used for precise delivery of medications or nutrients in trials, from chemotherapy drugs to insulin.
-
Spirometers
Devices measuring lung function, used in clinical trials involving respiratory diseases like asthma and COPD.
-
ECG/EKG Machines
Widely used in trials to monitor heart function and detect abnormalities, especially in cardiovascular studies.
Integrate with
Application Specific Systems
-
Laboratory Information Management Systems (LIMS)
Manage, track, and document laboratory processes, including receiving, testing samples, analysing, and managing resulting data.
-
Electronic Health Record (EHR) Systems
Provide comprehensive records of a patient’s medical history. Used in clinical research for patient recruitment, data collection, and post-study follow-up.
-
Interactive Response Technology (IRT) Systems
Also known as Randomisation and Trial Supply Management (RTSM) systems, used for patient randomisation, drug supply management, and managing blinding protocols in clinical trials.
-
Digital Biomarker Integration
Incorporates data from digital devices into clinical trials or healthcare applications for improved disease monitoring, diagnosis, prediction, and treatment optimisation.
-
Investigator Portals
Secure online platforms providing investigators with access to essential study documents, data, and tools to enhance communication, efficiency, and information accuracy.
-
Electronic Trial Master File (eTMF)
A digital system storing all documents associated with a clinical trial, ensuring regulatory compliance through efficient document management and audit trails.
-
Pharmacovigilance Systems
Systems used to detect, assess, understand, and prevent adverse drug effects, thereby improving patient safety and informing regulatory decisions about drug approval and use.
-
Virtual Reality
VR enables immersive experiences and simulations, enhancing patient engagement, therapeutic interventions, and data collection accuracy.
-
eSignature Vendors
Our eConsent module is designed to meet specific country requirements by integrating various eSignature providers. Experience a seamless and compliant consent processes tailored to each jurisdiction, ensuring regulatory compliance.
Let's connect and collaborate
Contact us and we will be happy to show you how Your Research makes a difference in conducting clinical research more efficient and personalized for everyone that is involved.