Usability testing
Enhancing the User Experience in Clinical Research
At Your Research, we are committed to revolutionising the field of clinical research by developing innovative solutions that improve the overall experience for both researchers and participants. To achieve this goal, we place great emphasis on usability testing, which allows us to gather invaluable feedback and insights from users.


Actively involving
Meeting the needs of our users
In today’s fast-paced world, where technology plays a central role in our lives, we find it crucial to prioritise user experience in every aspect of our product development. Usability testing is a fundamental process that helps us ensure our clinical research solutions are user-friendly, and meet the needs of our users.
Through usability testing, we gather feedback from a diverse group of participants, including researchers, clinicians, and study participants. This feedback enables us to identify potential issues, understand user behaviour, and make informed design decisions to enhance the overall user experience.
Feedback results
Clinical Research Participant App
Our clinical research participant app has undergone rigorous usability testing to ensure it provides a seamless and empowering experience for study participants. Here are some of the key results we have achieved:
Intuitive interface
Through usability testing, we are continuously improving the app's interface, making it intuitive and easy to navigate for users of varying technological backgrounds. Participants reported feeling confident and comfortable while using our app.
Enhanced engagement
Usability testing allowed us to identify areas for improvement in terms of participant engagement. By incorporating participant feedback, we have successfully implemented features that promote active involvement, such as personalised notifications, progress tracking, and interactive modules.
Streamlined communication
Our usability testing efforts have led to significant enhancements in communication features within the app. Participants can now easily connect with researchers, receive study updates, and access support, fostering a stronger sense of connection and trust throughout their participant research journey.

Feedback results
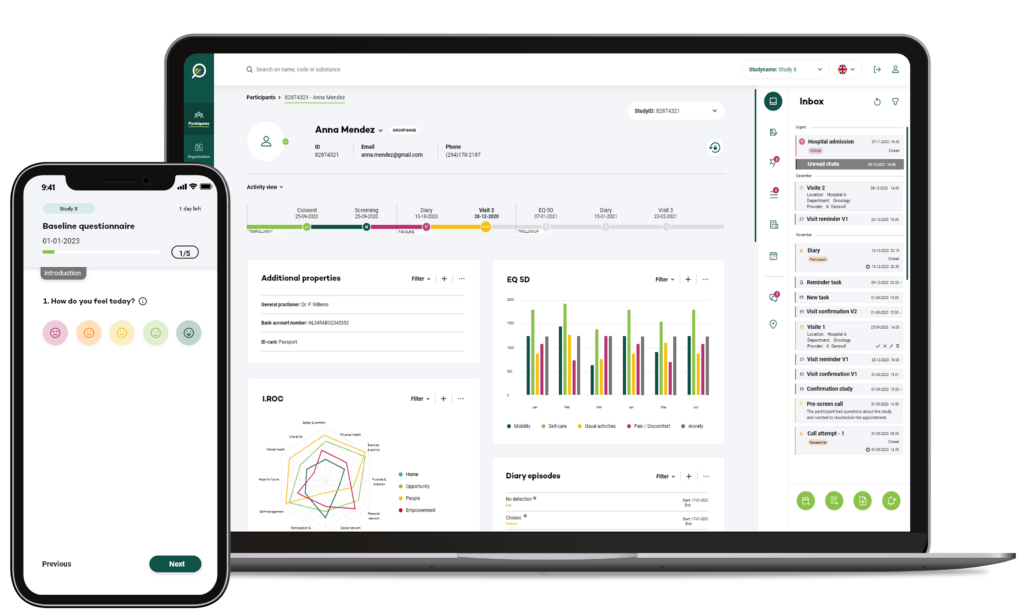
Study team research portal
The Your Research portal, designed for researchers and study coordinators, is an important instrument to manage clinical research processes and interactions with participant efficiently. This portal is developed through researcher led feedback. Here are the notable results we have achieved:
Simplified workflow
Usability testing allowed us to identify pain points and areas of complexity in the initial design. Through iterative improvements, we have streamlined the workflow, enabling researchers to efficiently manage their studies, track participant progress, and access essential study-related information.
Enhanced collaboration
Participants in our usability testing sessions emphasised the importance of seamless collaboration within research teams. We have incorporated their feedback to create features that facilitate real-time communication, data sharing, and task delegation among study team members, fostering a collaborative research environment.
Data visualisation
Our usability testing efforts have also focused on enhancing the presentation of study data within the web portal. Researchers now have access to comprehensive and visually appealing data visualisations, empowering them to gain deeper insights into study outcomes and trends.
Let's connect and collaborate
The results of our usability testing for the clinical research demonstrate our commitment to enhancing the user experience and revolutionizing the field of clinical research.
We invite you to join us on this exciting journey as we continue to innovate and empower researchers and study participants alike.
