Best in-class technology; unparalleled service
ePRO upgraded
Electronic Patient-Reported Outcomes (ePRO) revolutionize clinical trials by leveraging cutting-edge digital devices and software applications to capture critical patient-reported data directly from study participants.
Imagine participants effortlessly sharing real-time insights about their symptoms, quality of life, and treatment satisfaction—all from the convenience of their own devices. This innovative approach transforms the patient experience and enriches the research process, providing a treasure trove of valuable data.
At Your Research, our state-of-the-art ePRO system, a CE-marked medical device, boasts an extensive library of validated scales. It offers a personalized experience for subjects and empowers researchers with real-time data insights, enabling informed decisions and proactive monitoring throughout the trial. Embrace the future of clinical trials with ePRO and unlock the full potential of patient-driven research.
Avoid delays, deliver high-quality data, and retain participants with Your Research ePRO.
* * * * *
4,5/5 user reviews
Read More
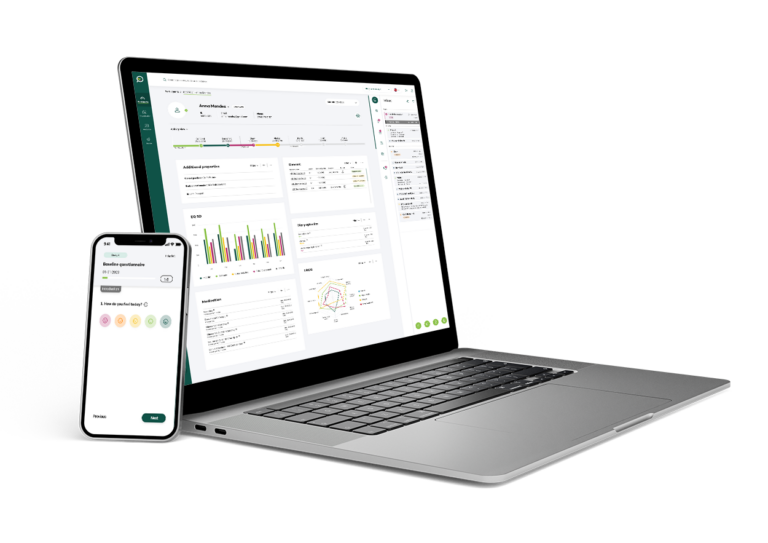
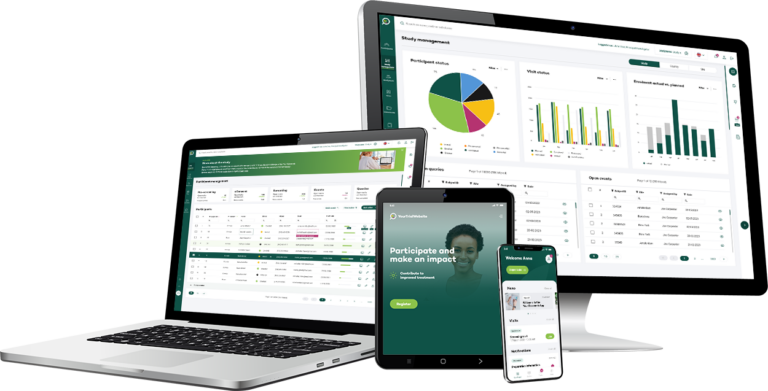
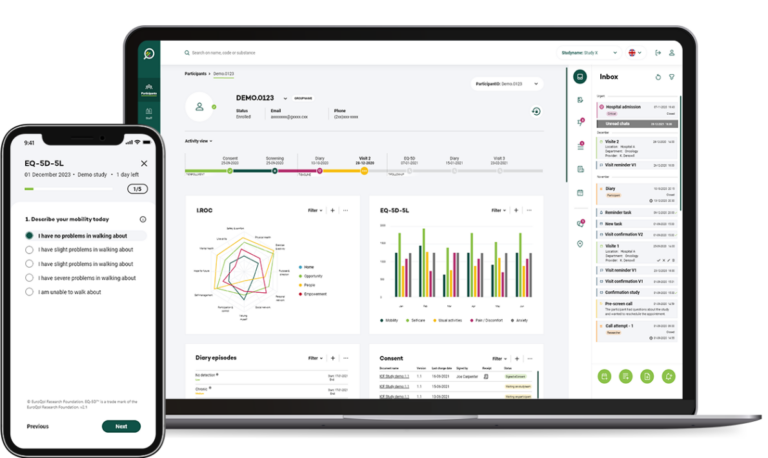
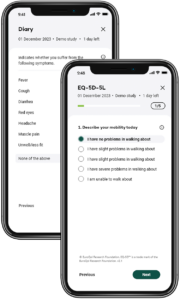
Key features
Enhance the entire patient journey for all stakeholders
We distinguish ourselves by supporting everyone involved in the trial to look ahead, intelligently guiding daily operations towards trial optimisation, with a focus on participant retention and the collection of high-quality data throughout the participant journey.
Real-Time Data Collection and Analysis
Our ePRO system enables real-time collection of patient-reported outcomes, ensuring that data is instantly available for analysis. This immediate access allows researchers to monitor patient progress, identify trends, and make timely decisions that can enhance the trial's efficiency and responsiveness.
Enhanced Patient Engagement and Compliance
By utilising our user-friendly digital interface, this ePRO systems make it easier for patients to report their symptoms and experiences regularly. Automated reminders and intuitive design help maintain high levels of patient engagement and compliance, reducing data gaps and ensuring more accurate and comprehensive data collection.
Robust Data Security and Privacy
Your Research ePRO is designed with advanced security measures to protect sensitive patient information. These systems comply with regulatory standards such as GDPR and HIPAA, ensuring that data is encrypted, securely stored, and accessible only to authorised personnel. This commitment to data security fosters trust among participants and regulators as it maintains the integrity of the clinical trial.
Global compliance
Your Research complies with international standards and regulations, ensuring data security and privacy across all operational regions.





From self to full service
Streamline service delivery to minimize risks and expedite startup
Support everyone involved in the trial to look ahead; intelligently guide daily operations towards trial optimisation; focus on participant retention and the collection of high-quality data throughout the participant journey; all this with Your Research ePRO.
Validated questionnaires
Use our pre-validated questionnaire library and existing relations with license holders
Translations
Our integrated network of translators will support with translation of questionnaires and communication information with participants.
IRB submission support
Our ePRO system is designed to meet regulatory standards, such as those set by the FDA, GDPR, and HIPAA.
By using our ePRO platform, researchers can demonstrate to the IRB that the study is in full compliance with relevant data protection and privacy regulations, which is a critical component of the ethical review process.
Device provisioning
Your Research provides pre-configured devices to patients for consistent performance and controlled study environments, as well as supports user owned devices (BYOD)
Training and support
Around-the-clock assistance to address technical issues and user inquiries. Training for clinical staff to ensure they are proficient in using Your Research and guide patients.
Beyond ePRO
Elevate the whole patient journey by adding integrable software modules
Fully integrated modules that make a difference and will have a direct positive impact on trial outcomes.
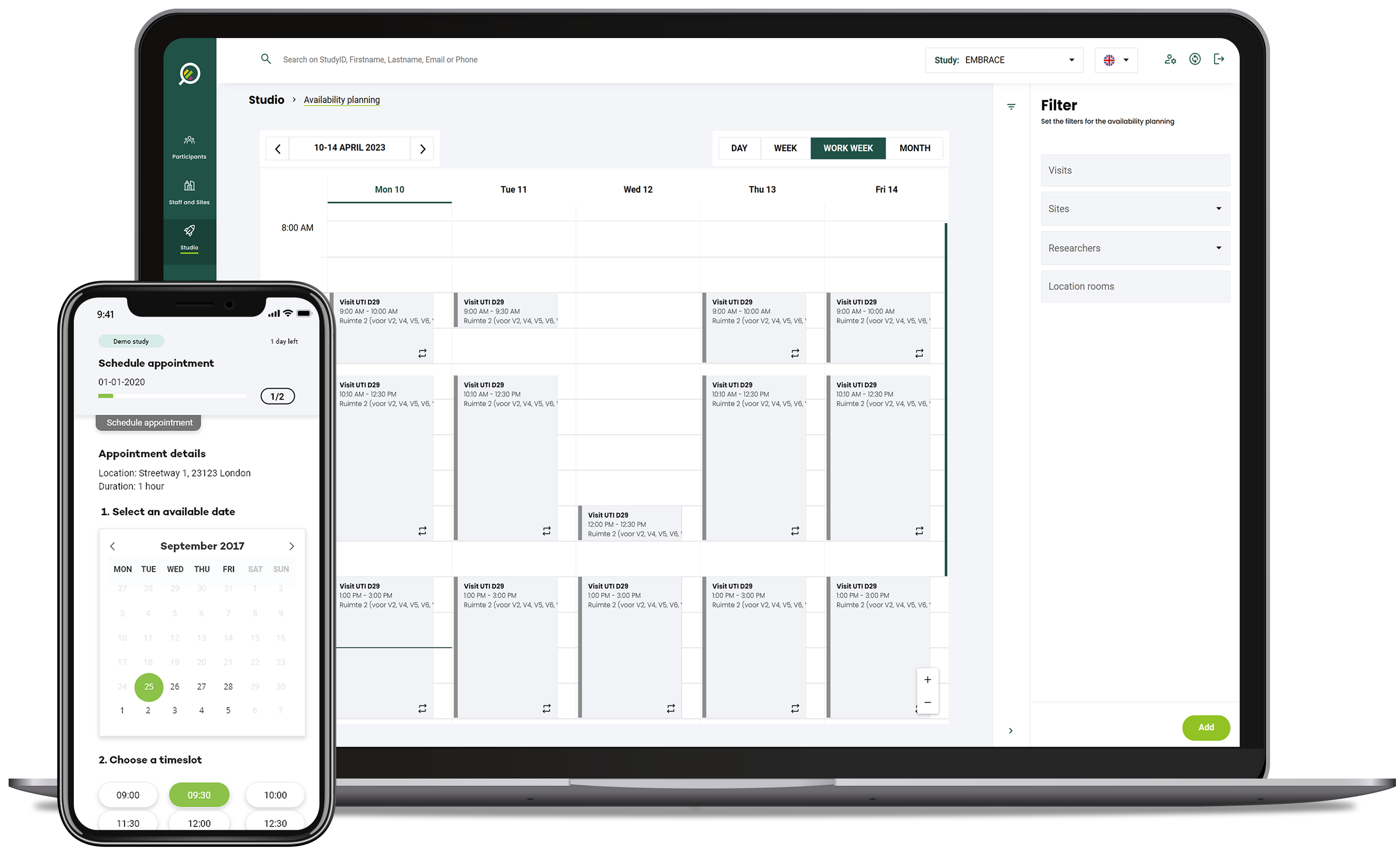
Visit scheduling
Real-time insights into protocol adherence and assistance in scheduling visits according to protocol, while reducing no-shows through proactive participant notifications.
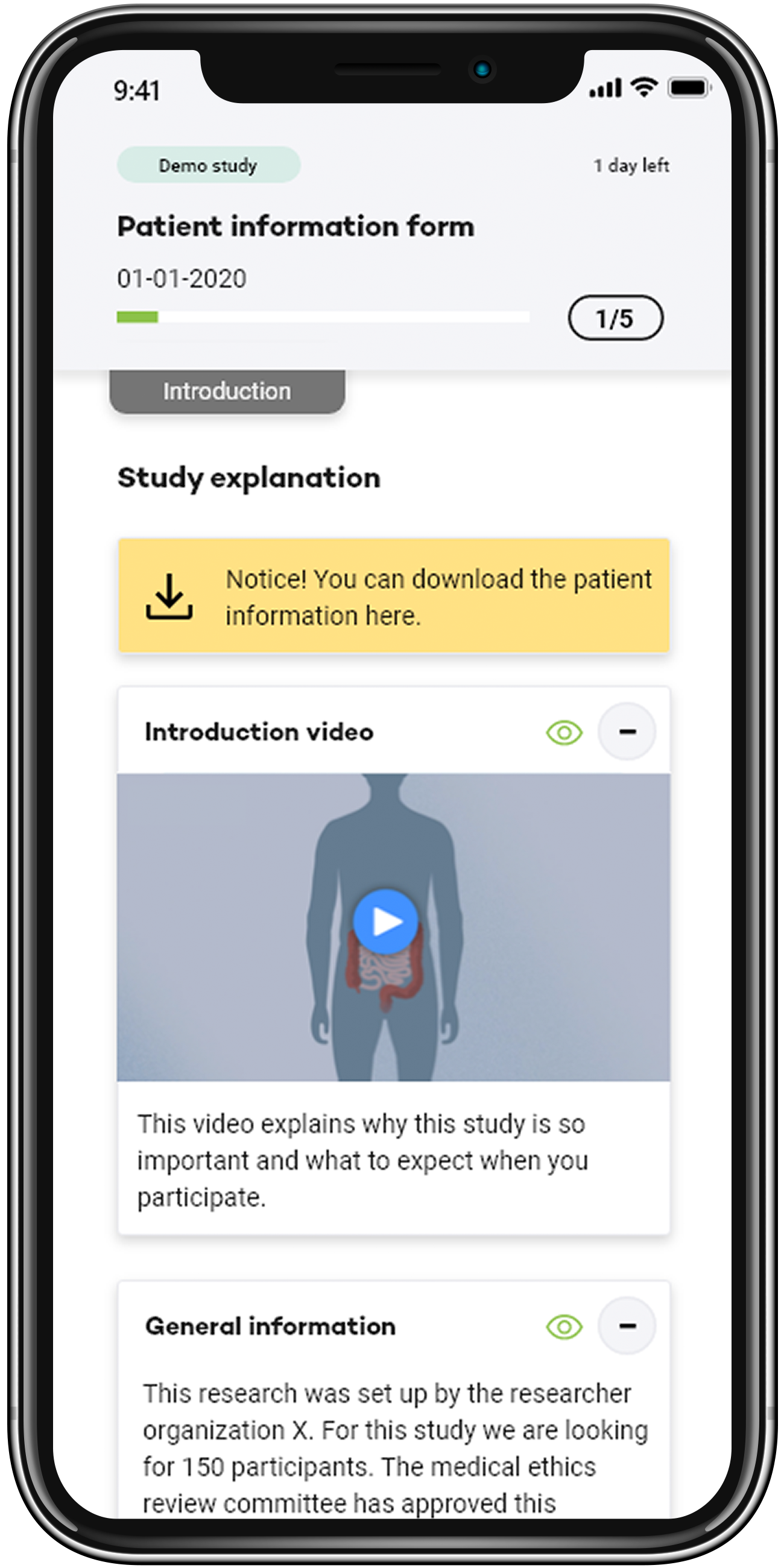
eConsent
eConsent streamlines the informed consent process through digital means, enhancing understanding and facilitating remote participation with interactive, easily accessible information.
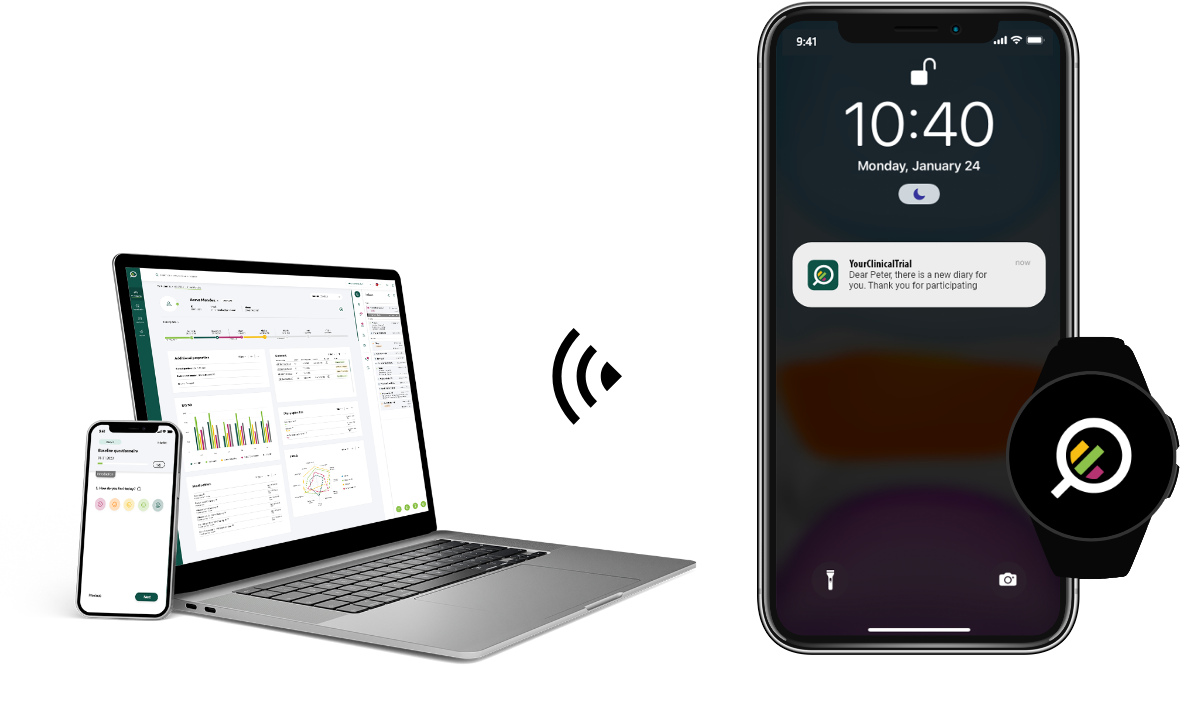
Connected devices
Collect vital values directly through devices such as blood pressure monitors, scales, SpO2 sensors, and activity trackers in Your Research, for real-time monitoring and improvement of patient outcomes.
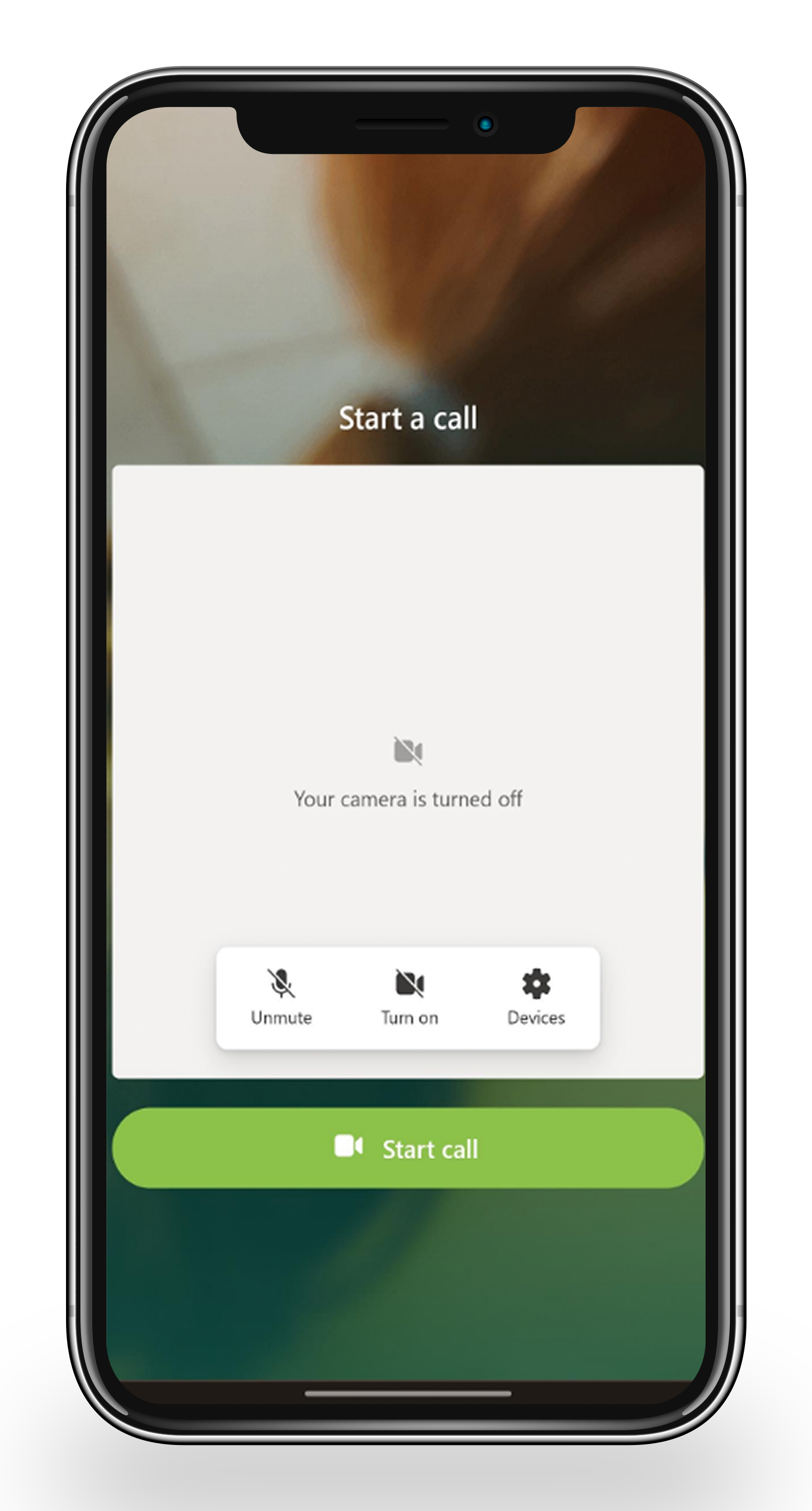
Televisits
Reduce participant burden by enabling remote visits through video calling or homecare nurse support, allowing interactions from home without the need for travel.
Experience efficient trials
Contact us and we will be happy to show you how Your Research makes a difference in conducting clinical research, making research more efficient and personalised for everyone involved.
