Best in-class technology; unparalleled service
eCRF
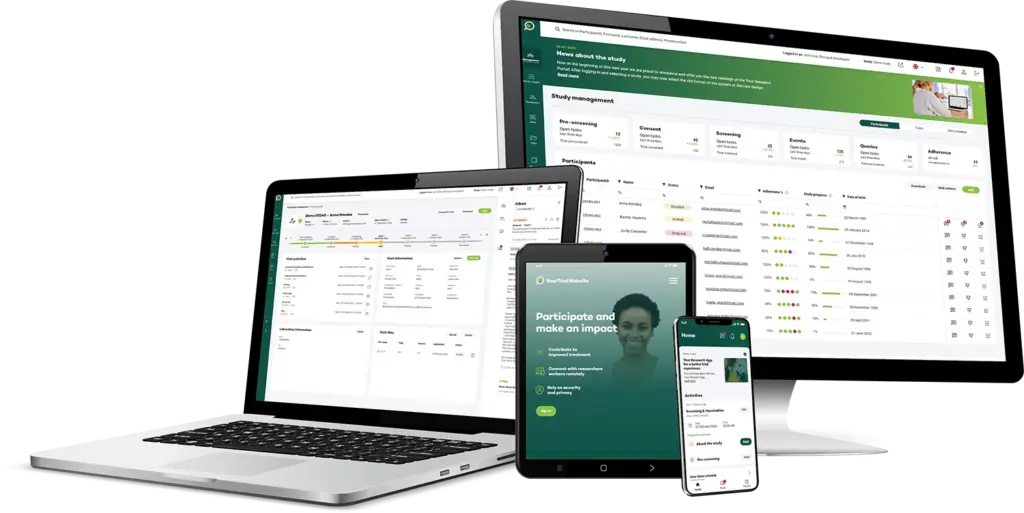
Your Research’s eCRF module is designed to capture various types of data, including demographics, medical history, laboratory results, adverse events, medication details, and other relevant information.
Your Research’s eCRF module is built to collect a wide range of data, including demographics, medical history, lab results, adverse events, and medication details.
The module ensures that all relevant trial information is captured efficiently and stored securely for easy access and analysis.
By capturing diverse and detailed participant data, the eCRF module supports more accurate trial outcomes and better-informed decision-making.

Key features
Optimal Secure Data Capture
Your Research’s eCRF module is designed to elevate data capture and management in clinical trials. With advanced features that streamline data collection, ensure accuracy, and integrate seamlessly with other eClinical tools, our eCRF module supports efficient and secure trial operations. Explore the top key features that make our eCRF solution stand out.
Intuitive design that simplifies data entry and ensures easy navigation for users.
Built-in checks to validate data at entry, reducing errors and enhancing data accuracy.
Flexible templates to adapt to various study requirements, allowing the collection of specific data types relevant to the trial.
Robust security protocols to ensure data protection and compliance with regulatory standards.
Seamless integration with other eClinical tools for streamlined data flow and comprehensive trial management.
Other Data Capture modules
Your Research facilitates efficient data management, organisation, and analysis, enabling study teams to smartly and proactively access and analyse patient-reported data more easily.





