Visit Scheduling
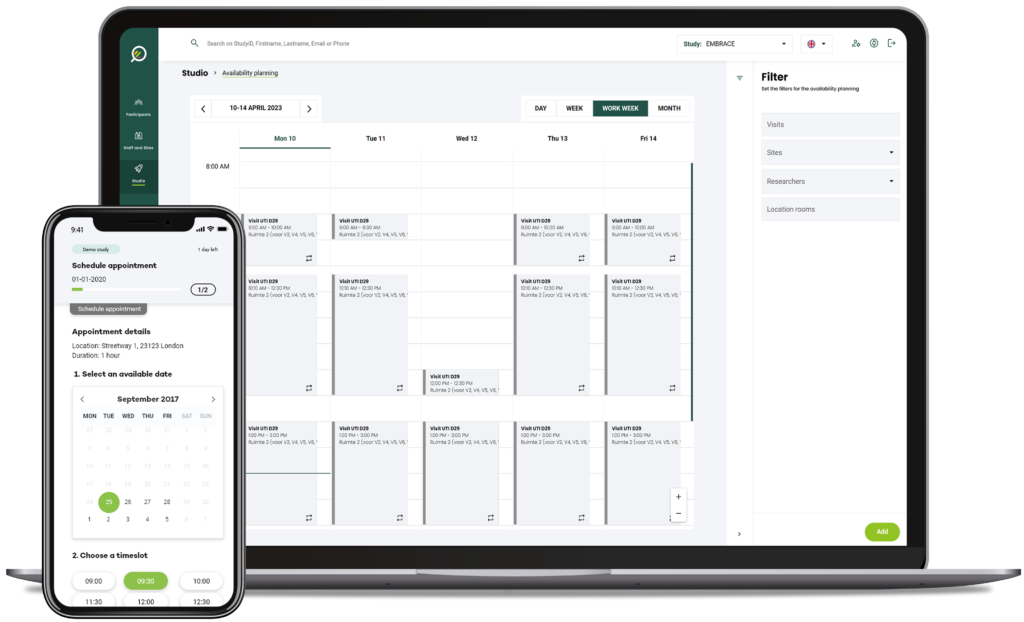
Easily set up virtual and in-person meetings, by pre-defining your availability and have prospective participants select a convenient time for their pre-consent or consent and other visits, depending on study protocol requirements.
A researcher simply fills in their availability on the scheduler, then when a prospective participant completes the study specific contact form, is prompted to select a specified date for their visit.
This can best be achieved through the Your Research App but can also be done from any digital device with an internet connection.
Key features
Predefine your availability
Experience the unparalleled efficiency of our Visit Scheduler module.
Predefine study visits in accordance with the study protocol, your and your facilities availability, and let participants select their preferred time to meet.
Automated Scheduling
Electronic visit scheduling enables automated scheduling of participant visits based on predefined criteria and visit windows.
The system can automatically assign suitable time slots, taking into account factors such as participant availability, study protocol requirements, and site capacity, simplifying the scheduling process for researchers.
Reminder and Notification System
Electronic visit scheduling incorporates a reminder and notification system that sends automated reminders to participants about upcoming visits. These reminders can be delivered via email, text messages, or through a dedicated participant portal, ensuring participants are well-informed and prepared for their scheduled visits.
This feature helps reduce no-show rates and improves participant adherence to the study protocol.
Participant Self-Service
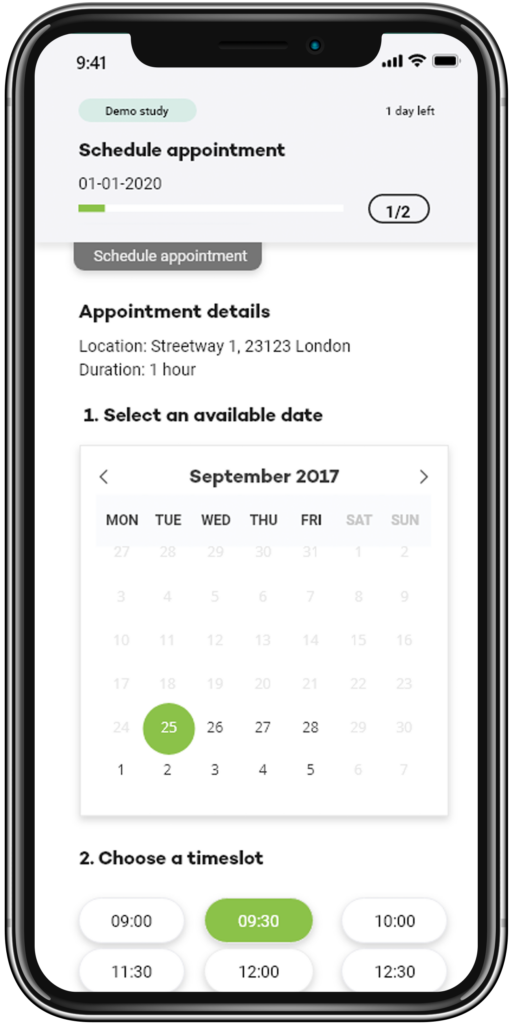
The Your Research electronic visit scheduling module provides a participant self-service portal, allowing participants to view their scheduled visits, reschedule appointments if needed, and update their availability.
This feature empowers participants by giving them control over their visit schedule, making it more convenient for them to manage their participation in the clinical trial.
It promotes participant engagement and satisfaction while reducing the administrative burden on study coordinators.
Benefits and value
Automation improves participant convenience
Visit scheduling allows for real-time tracking of participant progress and visit completion, enabling researchers to promptly address any deviations or issues, ultimately improving data accuracy and trial integrity.
Increased Efficiency
Electronic visit scheduling in clinical trials streamlines the process of scheduling and managing participant visits, reducing the administrative burden on both researchers and participants.
It allows for automated scheduling, reminders, and notifications, ensuring that visits are scheduled promptly and participants are well-informed, resulting in improved efficiency and time management.
Improved Engagement
Electronic visit scheduling enhances participant engagement by providing convenient and accessible options for scheduling and managing visits.
Participants can easily view and select available time slots, reschedule appointments if needed, and receive automated reminders and notifications. This level of convenience and flexibility encourages active participation and reduces the likelihood of missed visits, leading to improved adherence and data collection.
Enhanced Protocol Compliance and Data Accuracy
With electronic visit scheduling, researchers can closely monitor protocol compliance and ensure accurate data collection.
The system can enforce visit windows and protocol-specific requirements, preventing missed or delayed visits.
Usecases
Streamline visit bookings
Discover how our Visit Scheduler module optimises clinical trials and academic research, enhancing data collection and participant comprehension.
Case Study: eConsent Implementation – A Necessary Innovation and Optimisation in the Protea Paediatric Trial
Technical specifications
Integrate and scale in a secure environment
Integration Capability
Scalability and Performance
Security and Compliance
- Ability to integrate with other clinical trial systems, such as electronic data capture (EDC) systems, electronic health records (EHRs), or participant management systems.
- Integration allows for seamless data exchange and synchronisation, ensuring accurate and up-to-date visit schedules across different platforms.
- Able to handle a large volume of participants and visits efficiently.
- Robust performance capabilities to handle concurrent scheduling requests and ensure smooth operation even during peak usage times.
- Scaleable to accommodate the varying needs of different clinical trials, from small-scale studies to large multi-centre trials.
- Meets strict security and compliance requirements.
- Has appropriate data encryption measures, secure access controls, and comply with relevant data protection regulations: HIPAA or GDPR compliant.
- Undergoes regular security audits and assessments to ensure the protection of participant and study data.
Related modules
Along the participant journey
With Your Research, you have the flexibility to utilize stand-alone modules, integrate them with your existing software vendors, or connect them to other modules within Your Research to enhance the participant journey.
Make study visit planning easier for everyone
Contact us and we will be happy to show you how Your Research makes a difference in conducting clinical research, making research more efficient and personalised for everyone involved.


