Introduction
The assessment of symptoms in neurodegenerative diseases such as Huntington’s disease (HD) presents unique challenges.
Symptom monitoring in HD primarily relies on periodic in-person clinical assessments, including the administration of clinician-rated outcomes (which depend on the experience and expertise of the rater) and patient-reported outcomes (1, 2). Such as, these methods do not measure diurnal fluctuations, lack information on contextual aspects and may be subject to recall bias (1, 3).
To address this gap, this new study leverages Your Research’s electronic Patient Reported Outcomes (ePRO) via the MUMC Research App, utilising the experience sampling method (ESM) to collect more comprehensive data.
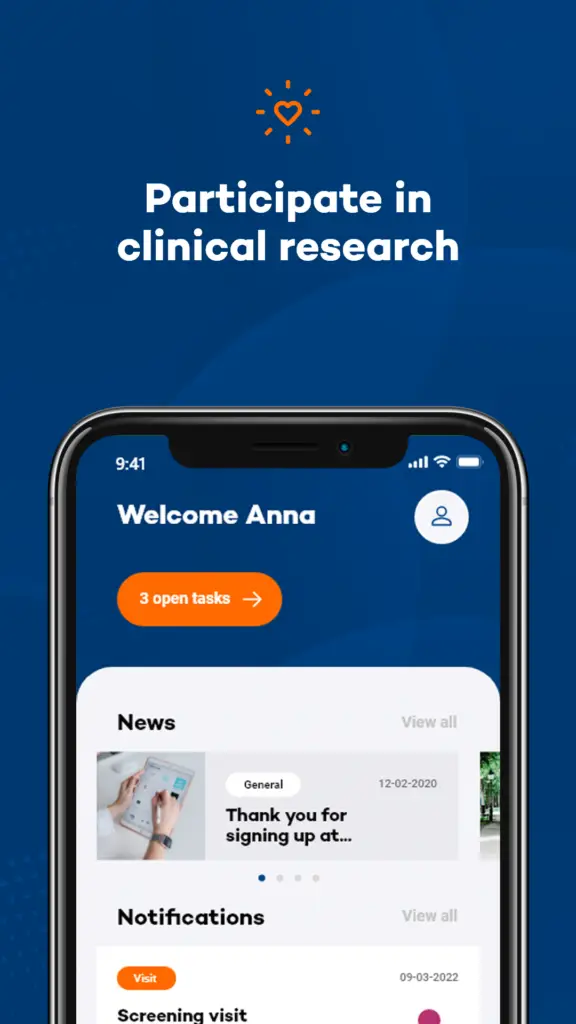
What is Being Investigated?
The study focuses on two primary areas:
- Daily Fluctuations in Symptom Severity: The core objective is to monitor and analyse how symptoms of Huntington’s disease vary throughout the day. This includes identifying patterns and peak periods of symptom severity.
- Environmental and Situational Influences: Researchers are also interested in understanding how different environments and situations affect symptom severity. By correlating symptom data with contextual information, the study aims to uncover potential triggers or mitigating factors that influence the disease’s manifestation.
Using the insights gained, the module can be refined and optimised for broader application, enhancing its utility in the clinical management of Huntington’s disease.
This study aims to:
- Assess the feasibility and validity of a newly developed ESM questionnaire for specific use in HD patients across different disease stages.
- Explore the usability of this ESM for detecting associations between baseline characteristics, motor symptoms, affective states and contextual factors on both an individual and a group levels in HD patients. By correlating symptom data with contextual information, the study aims to uncover potential triggers or mitigating factors that influence disease manifestation.
If the hypothesis is confirmed, ESM could be used for the following clinical applications:
- ESM can from the basis for initiating or adjusting of patient-centred treatment, e.g. by providing patient-specific advice on activities and lifestyle factors, or by targeting specific symptoms that appear to play a role in the symptom network and can be used alongside commonly used outcome measures to provide information on the fluctuating severity of symptoms over time.
- In research, this method can be used to evaluate the effectiveness of both pharmacological and non-pharmacological interventions.
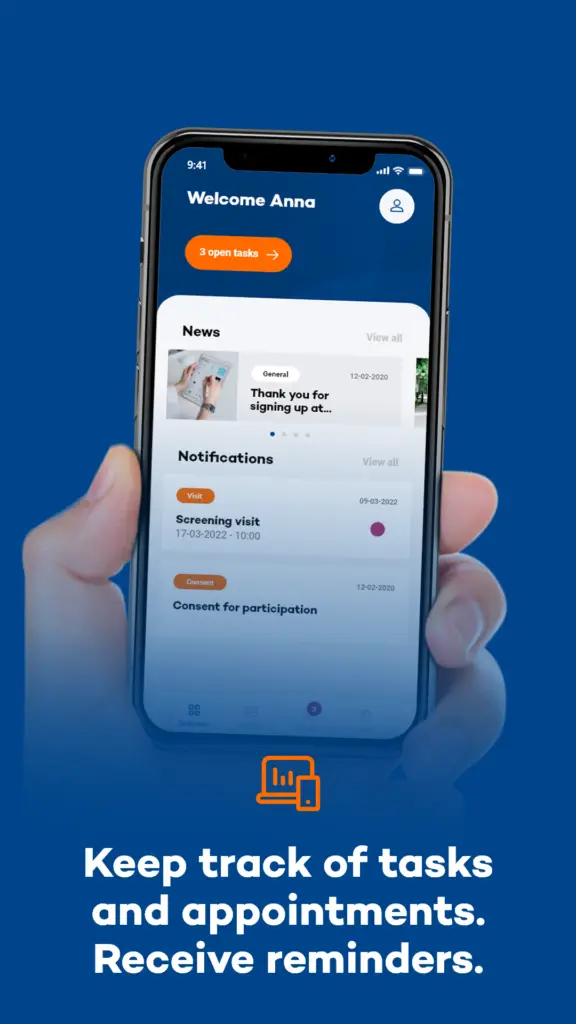
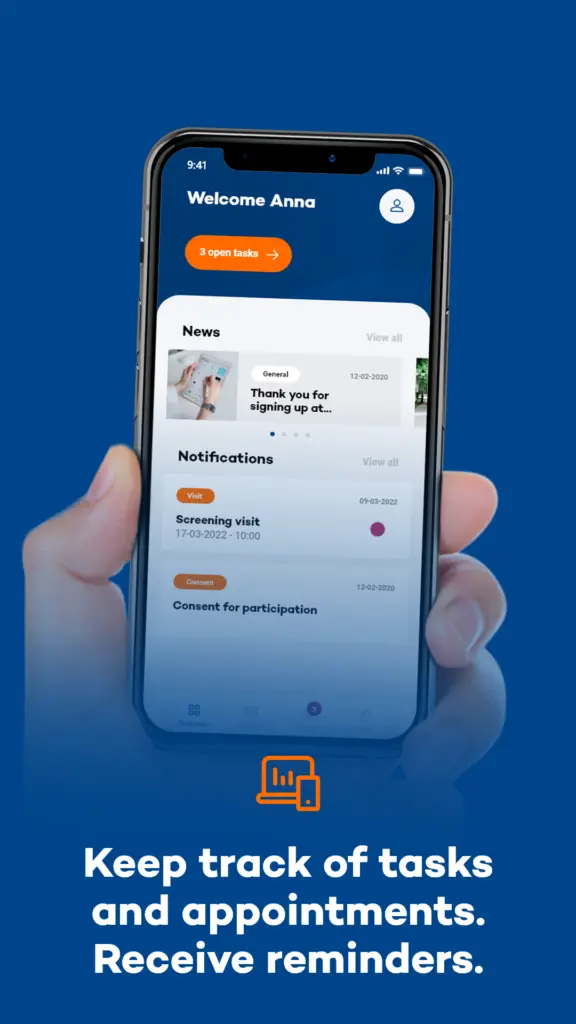
How is the Research Conducted?
Participants in the study will use the MUMC Research App for a period of one week. The app is designed to prompt users with a sound and text message, referred to as a beep, six times a day at a random time slot. Upon receiving the beep (push notification), participants are expected to respond immediately, within a maximum of 20 minutes, to a series of questions about their current (motor and non-motor) symptoms and situation, provided it is safe to do so. If the beep is missed, the opportunity to record data at that moment is lost after 20 minutes.
This high-frequency sampling approach ensures a rich dataset that captures the variability and context of symptoms in real-time. Importantly, all collected data is anonymized, with researchers only accessing non-identifiable information, thereby ensuring participant privacy.
What is Expected of Participants?
Participants are required to:
- Respond Promptly: Answer the app’s questions immediately upon receiving the beep, regardless of their location or activity, to ensure data accuracy and reliability.
- Commit for One Week: Engage with the app consistently for the entire duration of the study period to provide comprehensive data.
- Consent to Data Use: Sign a consent form authorising the use of their anonymised data for research purposes.

Conclusion
The integration of Your Research’s ePRO via the MUMC Research App represents a significant advancement in Huntington’s disease research. By moving beyond static, clinic-based assessments and embracing the dynamic experience sampling method, this study aims to generate a deeper understanding of symptom variability and its triggers. This approach not only holds promise for improving individual patient care but also contributes to the broader knowledge base needed to combat Huntington’s disease more effectively.
Through this innovative use of technology, MUMC researchers hope to pave the way for more responsive and personalised treatment strategies, ultimately enhancing the quality of life for those affected by Huntington’s disease.
About Maastricht University Medical Centre
Maastricht University Medical Centre+ is a collaboration between Maastricht University Hospital and Maastricht University’s Faculty of Health, Medicine & Life Sciences. Dedicated to delivering exceptional care and advancing health in the region, MUMC+ integrates patient care, research, and education to achieve its mission. This study exemplifies MUMC+’s commitment to innovation and improving patient outcomes through cutting-edge research.

About the Maastricht UMC Research App
The Maastricht UMC Research App, managed by Maastricht UMC+, facilitates medical-scientific studies under the supervision of Maastricht UMC+. This app allows you to contribute to scientific research by participating as a test subject by:
- Registering Your Interest: Sign up to express your interest in participating in scientific studies, with no obligation to participate. You will be notified via the app if a suitable study is available.
- Joining a Study: Register for specific studies and we will contact you for an informative conversation. Participation is entirely voluntary.
Understanding Huntingtons Disease
Huntington’s disease (HD) is a progressive neurodegenerative disorder caused by a genetic mutation. It leads to the gradual breakdown of nerve cells in the brain, resulting in physical impairment, psychiatric and behavioural symptoms and cognitive decline. Generally, the first symptoms of HD start at 30-50 years of age, with a reported range of 2 to 85 years (6).
Symptoms typically begin between the ages of 30 and 50, but they can start earlier or later.
Key Aspects of Huntington’s Disease:
Genetic Basis: HD is inherited in an autosomal dominant pattern, meaning that a child of an affected parent has a 50% chance of inheriting the disease.
Symptoms: HD is characterised by involuntary movements (chorea), cognitive deterioration and neuropsychiatric symptoms, the latter of which can develop 10-15 years prior to clinical diagnosis (7, 8).
As the disease progresses, patients may experience more severe chorea, difficulty in speaking and swallowing, and severe cognitive decline.
Diagnosis: The diagnosis of HD is based on clinical evaluation and family history and can be confirmed by genetic testing. Clinical disease onset (manifest HD) begins with the development of motor symptoms as defined by the diagnostic confidence level of the Unified Huntington’s Disease Rating Scale (UHDRS) (9).
Management: While there is currently no cure for HD, treatments focus on managing symptoms and improving the quality of life. This includes medications to control physical and psychiatric symptoms, as well as supportive therapies such as physical, occupational, and speech therapy.
Research and Hope: Ongoing research aims to better understand the disease mechanisms and develop potential treatments. Clinical studies, such as those facilitated by the Maastricht UMC, are crucial in this endeavour, offering hope for future breakthroughs.
Through education, support, and innovative research, we strive to improve the lives of individuals affected by Huntington’s disease and move closer to finding a cure.
References
Your Research would like to express our gratitude to Ruben Andriessen for his significant contribution to this Case Study.
- Dorsey ER, Papapetropoulos S, Xiong M, Kieburtz K. The First Frontier: Digital Biomarkers for Neurodegenerative Disorders. Digit Biomark. 2017;1(1):6-13.
- Post B, Merkus MP, de Bie RM, de Haan RJ, Speelman JD. Unified Parkinson’s disease rating scale motor examination: are ratings of nurses, residents in neurology, and movement disorders specialists interchangeable? Mov Disord. 2005;20(12):1577-84.
- Broen MP, Marsman VA, Kuijf ML, Van Oostenbrugge RJ, van Os J, Leentjens AF. Unraveling the Relationship between Motor Symptoms, Affective States and Contextual Factors in Parkinson’s Disease: A Feasibility Study of the Experience Sampling Method. PLoS One. 2016;11(3):e0151195.
- Habets J, Heijmans M, Herff C, Simons C, Leentjens AF, Temel Y, et al. Mobile Health Daily Life Monitoring for Parkinson Disease: Development and Validation of Ecological Momentary Assessments. JMIR Mhealth Uhealth. 2020;8(5):e15628.
- Mulders AEP, van der Velden RMJ, Drukker M, Broen MPG, Kuijf ML, Leentjens AFG. Usability of the Experience Sampling Method in Parkinson’s Disease on a Group and Individual Level. Mov Disord. 2020;35(7):1145-52.
- Roos RA. Huntington’s disease: a clinical review. Orphanet J Rare Dis. 2010;5:40.
- van Duijn E, Craufurd D, Hubers AA, Giltay EJ, Bonelli R, Rickards H, et al. Neuropsychiatric symptoms in a European Huntington’s disease cohort (REGISTRY). J Neurol Neurosurg Psychiatry. 2014;85(12):1411-8.
- Duff K, Paulsen JS, Beglinger LJ, Langbehn DR, Stout JC. Psychiatric symptoms in Huntington’s disease before diagnosis: the predict-HD study. Biol Psychiatry. 2007;62(12):1341-6.
- Reilmann R, Leavitt BR, Ross CA. Diagnostic criteria for Huntington’s disease based on natural history. Mov Disord. 2014;29(11):1335-41.
- Joining a Study: Register for specific studies and we will contact you for an informative conversation. Participation is entirely voluntary.



