Introduction
At Your Research, we’re proud to be supporting the MOOD study led by Erasmus MC in Rotterdam. This innovative research aims to explore the early detection of mental health problems using a simple, app-based questionnaire. By offering recruitment services and multilingual capabilities, we ensure seamless participation for over 1,500 adults in Rotterdam, enhancing accessibility and data accuracy.
This is a pilot study that hasn’t been conducted before and could therefore provide new insights that contribute to public mental health.

Pioneering Mental Health Research with Advanced Technology
The MOOD study focuses on early detection—already a common practice in physical health—now being applied to mental health. Using the Your Research app, participants are invited to complete monthly questionnaires that help researchers gather insights into their mental well-being. Early detection can allow timely intervention and help prevent more severe disease in the future.
The study, conducted in multiple languages (Dutch, English, Turkish, Arabic, Polish and Spanish), engages a diverse population, ensuring inclusivity across the community.
Mental health, specifically depression, is a topic that still often carries a stigma. By offering the study in multiple languages, Erasmus MC hopes to reduce both this barrier and the language barrier.
With Your Research’s recruitment and multilingual solutions, the study is able to reach participants who may otherwise face language barriers, maximizing the inclusiveness and impact of the research.
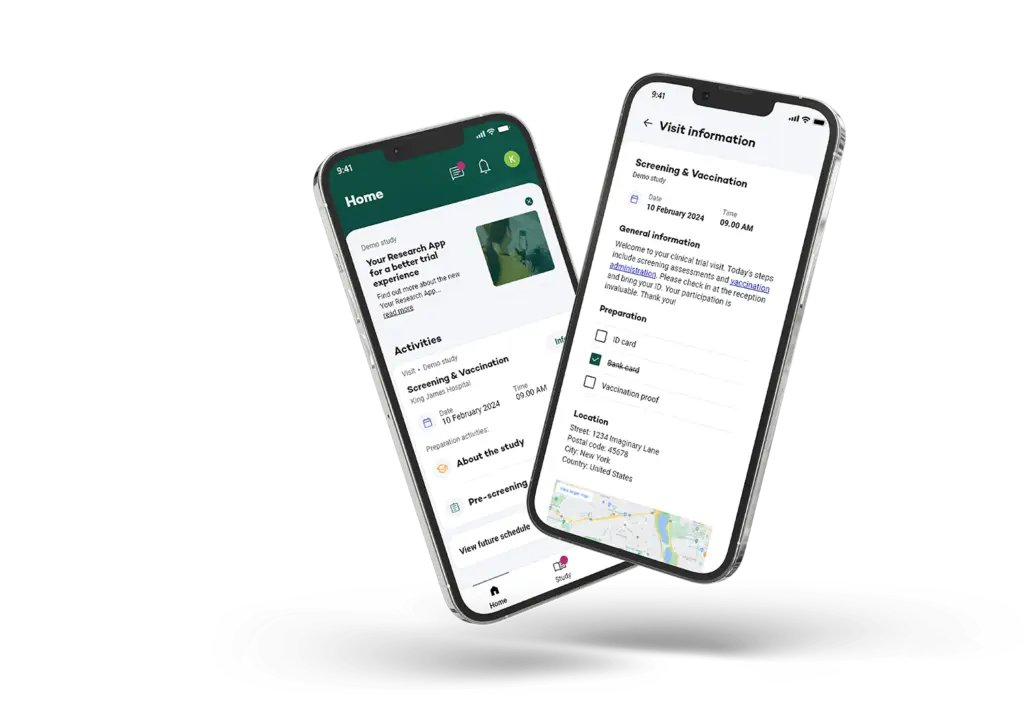
Supporting Recruitment, eConsent, Randomisation and Data collection with Multilingual app
Your Research plays a critical role in participant recruitment for the MOOD study, which is reaching out to Rotterdam residents and encouraging their involvement. The recruitment specific website is designed to help identify, pre-screen and onboard participants efficiently, even across large populations.
After registration participants can give electronically consent, via the eConsent module.
Consent is a very well regulated step in clinical trials and the Your Research eConsent module is adherent to all relative regulations regarding consent.
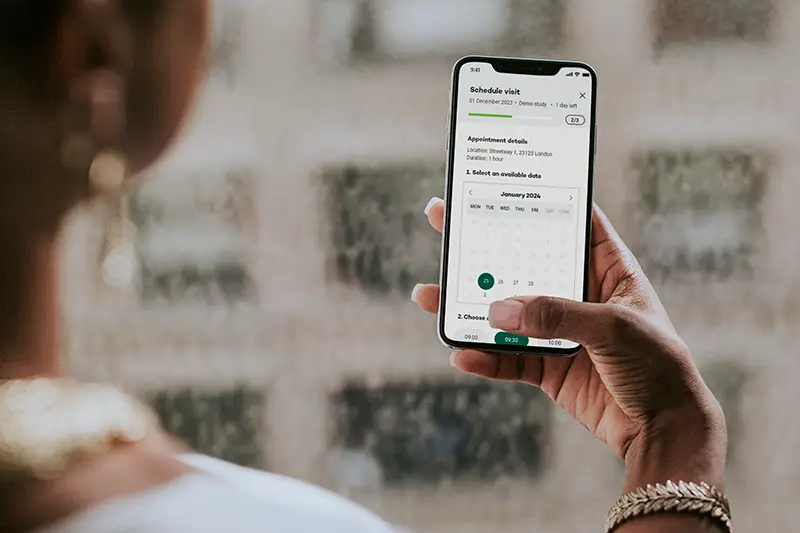
Before participants start with data collection they get randomized in 3 different groups, after this participants can complete their monthly check-ins in their preferred language (6 languages implemented), making it easier for non-Dutch speakers to take part in the study. This inclusive approach enriches the dataset and ensures that mental health research truly reflects the diverse community it aims to help.
Additionally, Your Research provides ePRO data collection services, featuring validated scales like the EQ-5D-5L and PHQ-9, along with support for study teams in patient reimbursement and participant communication.
The goal: A Positive Contribution to Society
This study has the potential to make a significant social impact by identifying mental health issues early and guiding residents to the appropriate resources. By participating, individuals help contribute to a greater understanding of mental health in the Rotterdam region and the Netherlands as a whole.
Over MOOD Studie – Engels




