Your Research empowers you to run your trials efficiently, predictably and with less risk by
Uniting workflows, guiding study teams and retaining participants.
A Holistic Approach
Navigate clinical trial processes and systems with ease
Navigating clinical trials demands more than technology; it requires an integrated system for simplified coordination. Your Research provides a comprehensive, centralized solution uniting workflows and system integrations, ensuring seamless communication and intuitive design to keep stakeholders informed and focused on their research.
Your Research’s coordinated umbrella framework integrates clinical trial processes and systems into one centralised solution, enhancing workflow management and ensuring a smooth transition between steps
Our core suite, featuring both a unified software solution and third-party integrations, offers seamless access to all necessary tools and data, simplifying complex trial coordination.
By aligning with a Unified Protocol, our solution ensures all stakeholders remain consistently informed and focused on the critical aspects of the trial, reducing miscommunication and enhancing efficiency.
Patient-Centric By Design
Insight and guidance along the Participant Journey
Your Research software is designed around the Participant Journey, from recruitment to visit scheduling and resource management.
Our solution supports participants with automated pre-screening, eConsent, and efficient visit scheduling, ensuring every step aligns with the trial protocol while reducing errors and saving time.
A suite of tools designed to attract, pre-screen, and onboard suitable participants through digital channels, optimising outreach and ensuring qualified candidates join the study.
A secure, digital consent solution that guides participants through study information and collects electronic signatures, ensuring informed consent with streamlined compliance and record-keeping.
A comprehensive suite of modules for managing participant scheduling, randomisation, and medication logistics, facilitating a seamless transition from consent to active study involvement, guiding researchers and retaining participants.
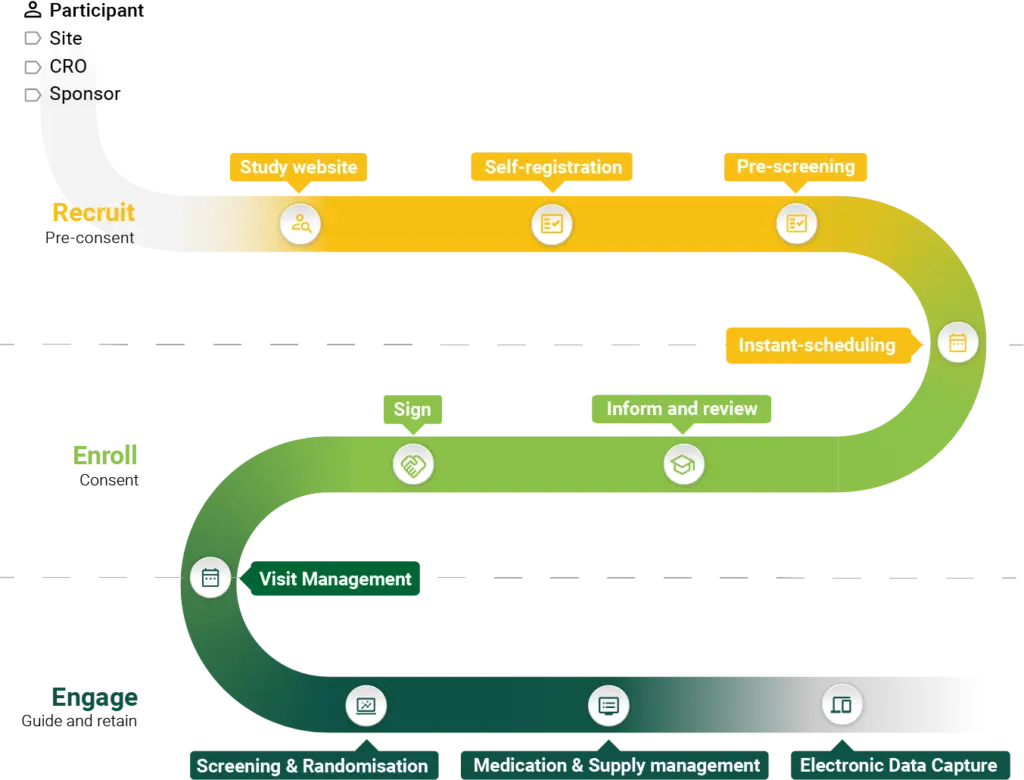
Solutions at a glance
Your Research provides innovative, fully integrated eClinical solutions designed to simplify and optimise the clinical trial process.
From recruitment and eConsent to real-time data capture and advanced analytics, our tools support every stage of the Participant Journey, ensuring accuracy, compliance, and efficiency.
Positive User Engagement
Exceptional user interaction and design
Our solution is praised for its ease of use and intuitive design. We’ve made it a priority to create a user-friendly environment for researchers and participants alike. This results in higher technology adoption rates and smoother trial execution.
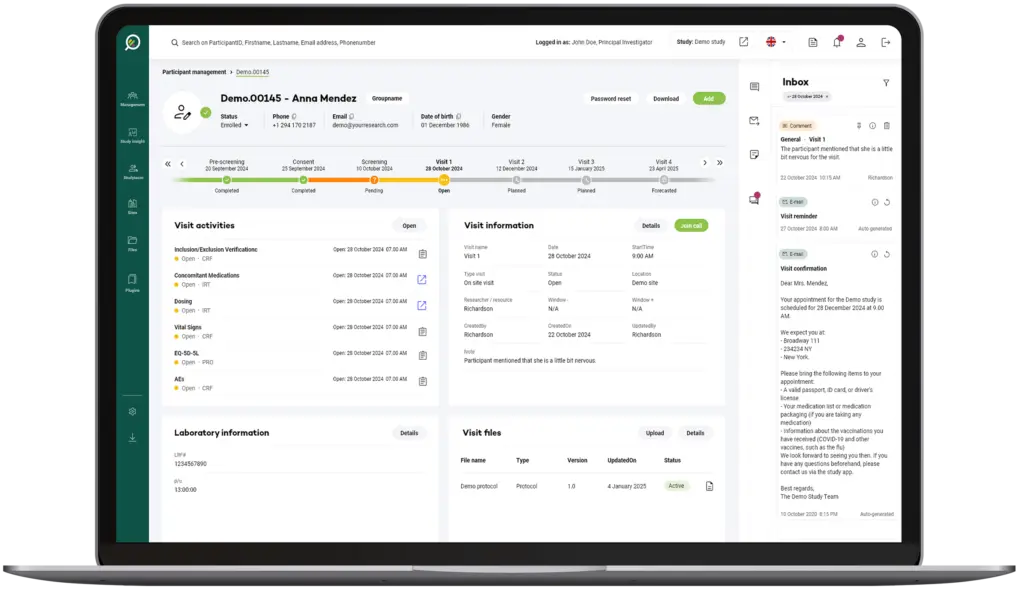
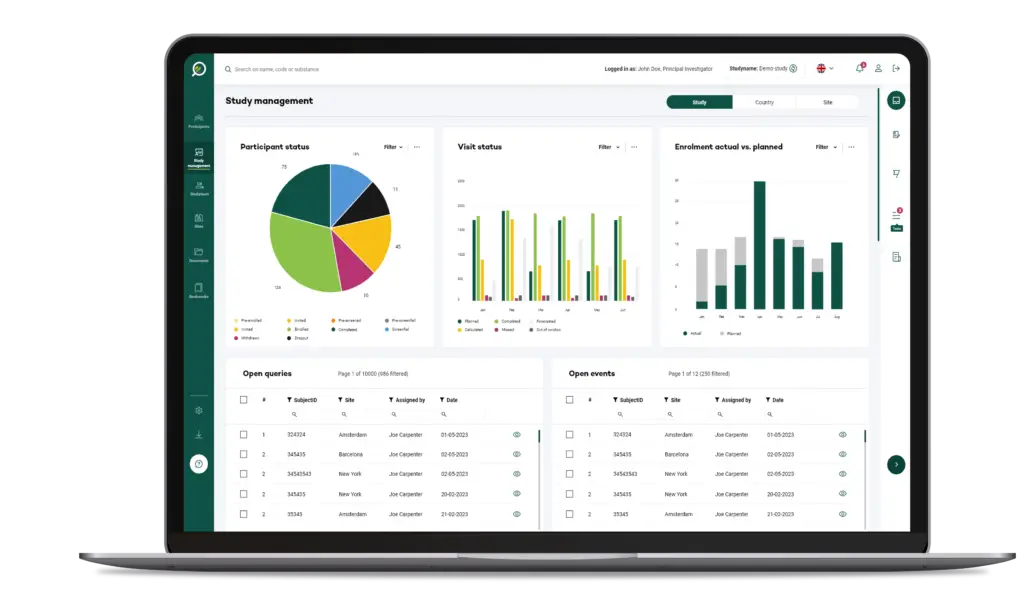
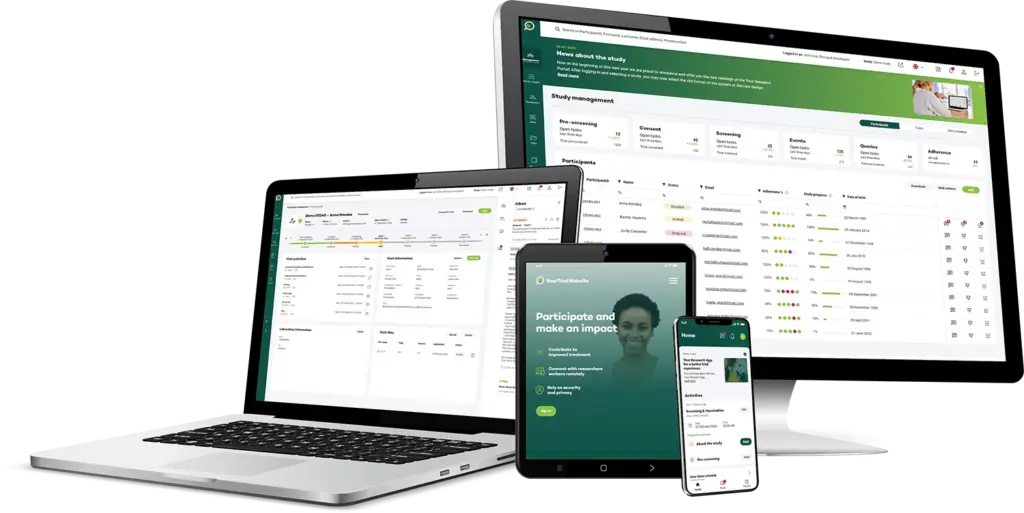
Your Research's software is designed to be simple and straightforward for users at all levels, whether they're researchers or participants.
By reducing the learning curve, the software allows users to navigate and use its features with minimal training.
This accessibility is especially important in clinical research, where users may have varying levels of technical expertise and need to perform tasks quickly and accurately.
The User Interface (UI) is structured to anticipate user needs and make essential functions easy to locate and use.
This intuitive layout means users can accomplish tasks like scheduling, data entry, or report generation in fewer steps, reducing frustration and time spent.
For participants, an intuitive design enhances engagement, as they can interact with the software without confusion, making it more likely they'll stay compliant with study requirements.
User-friendly software leads to higher adoption rates among users, as they are more willing to engage with a system that feels easy to operate.
For research teams, this means more efficient operations, as they encounter fewer obstacles when using the platform.
The improved User Experience (UX) also translates into smoother trial execution overall, with fewer errors, less downtime, and improved data quality, ultimately contributing to more successful trials.











