Introduction
The POS-ARI-PC study, initiated in 2021 under the ECRAID-Base project, represents a significant advance in primary care for acute respiratory infections (ARI). These infections, such as influenza and COVID-19, are a leading reason for patients visiting primary care sites such as general practices (GPs), urgent care, and paediatric centres. The study aims to better understand the diagnosis, treatment, and prevention of ARI while providing critical data that can improve patient care, reduce inappropriate antibiotic use, and inform future clinical trials.
With seven sites and 70 enrolled patients, Your Research is proud to contribute to this innovative project. Our eCOA services of participant management and secure storage of patient personal information supports the POS-ARI-PC study’s objective to deliver benchmark data on ARI management across different countries.

Patient Engagement: Enhancing the Participant Journey
ARI remains a significant global health challenge, with new pathogens often first detected in primary care. As the study progresses, it highlights four critical areas of focus:
Accurate Diagnosis and Complication Prediction
Swift identification of respiratory pathogens enables healthcare professionals to predict complications more effectively, thus ensuring timely interventions.
Antibiotic and Antiviral Stewardship
Ensuring appropriate prescribing decisions is vital in combating the global crisis of antibiotic resistance, particularly in primary care where most ARI cases are managed.
Optimising Care During Epidemics and Pandemics
Having a system that can quickly adapt to new outbreaks allows for improved management strategies in times of crisis, a necessity highlighted during the COVID-19 pandemic.
Prevention Through Vaccination
Understanding ARI trends assists in better planning vaccination campaigns, ultimately reducing the burden of infections.
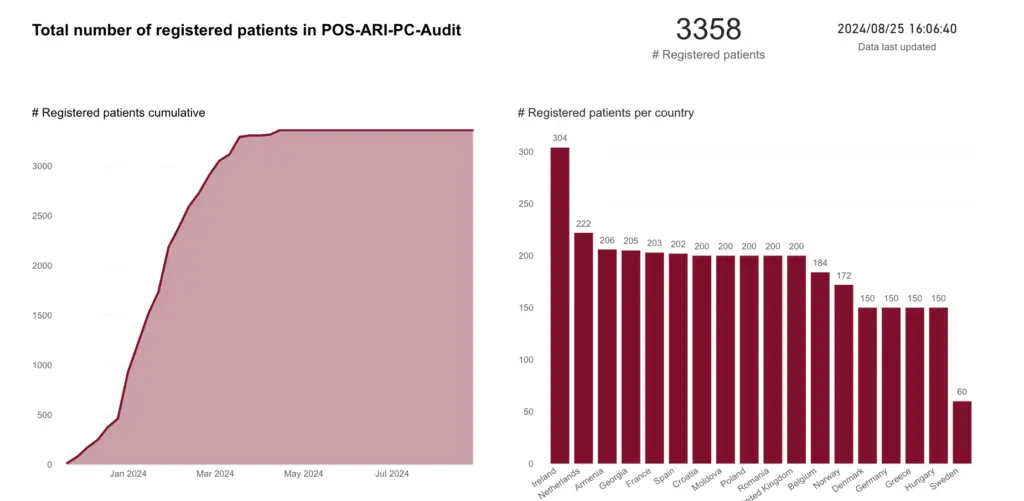
The audit conducted in late 2023 across 90 sites in 18 countries involved over 3,300 registered patients. This comprehensive collection of patient characteristics, treatment methods, and outcomes sheds light on how ARIs are managed in various healthcare systems.
Building a Future-Ready Research Infrastructure
One of the standout features of the POS-ARI-PC study is its focus on creating a “research-ready” infrastructure. This infrastructure is designed to facilitate quicker responses to future epidemics and pandemics by allowing new studies to be seamlessly “plugged in” without extensive regulatory delays. This flexibility supports more agile healthcare research, ultimately benefitting patients during global health emergencies.
Our participant journey focused technology underpins this infrastructure, streamlining data collection from patient interactions to lab results and reducing the time from trial initiation to actionable outcomes. This enhanced efficiency is critical for creating a scalable platform capable of supporting rapid clinical trials in response to emerging respiratory threats.

Patient Engagement: Enhancing the Participant Journey
Your Research’s involvement in the POS-ARI-PC study is a testament to our commitment to enhancing patient care, especially in the complex field of acute respiratory infections. By providing innovative eCOA services, we enable clinical sites to capture data seamlessly and contribute to a global understanding of ARI.
This partnership highlights how technology can drive real-world improvements in healthcare, from better diagnostic practices to optimized treatment protocols and ultimately, more efficient healthcare systems prepared for future challenges. As we look to the future, Your Research is proud to be part of a solution that helps healthcare systems remain resilient in the face of evolving respiratory pathogens.
Commitment to ARI Management
Your Research’s involvement in the POS-ARI-PC study is a testament to our commitment to enhancing patient care, especially in the complex field of acute respiratory infections. By providing innovative eCOA services, we enable clinical sites to capture data seamlessly and contribute to a global understanding of ARI.
This partnership highlights how technology can drive real-world improvements in healthcare, from better diagnostic practices to optimised treatment protocols and ultimately, more efficient healthcare systems prepared for future challenges. As we look to the future, Your Research is proud to be part of a solution that helps healthcare systems remain resilient in the face of evolving respiratory pathogens.