Introduction
Retaining recruited participants for clinical research is a critical aspect of advancing medical knowledge and improving patient outcomes. However, the process is riddled with challenges that often hinder the progress of research studies.
In a recent poll conducted among more than 27,000 life science professionals, several key issues emerged, shedding light on the difficulties faced in participant recruitment.
This article explores four major challenges:
- Logistic & practical aspects;
- Perceived Lack of efficacy;
- Personal health issues;
- Safety & side effect concerns;
We also delve into potential solutions to overcome these obstacles.
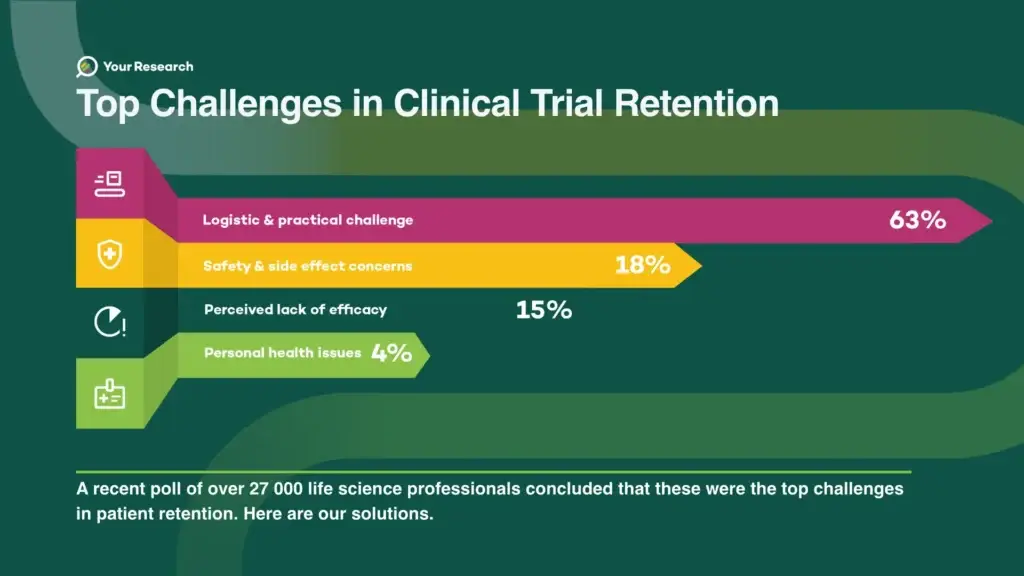
Logistic & Practical Challenges 63%
The majority of respondents to the poll emphasised that logistical and practical aspects pose significant challenges in retaining participants in clinical trials. This, we understand, is due to their direct impact on the participants’ ability to adhere to study protocols.
Challenge
Participants often face logistical hurdles such as transportation issues, time constraints, and scheduling conflicts, which can disrupt their regular participation in clinical trials. Practical concerns, such as the burden of frequent clinic visits and complex study procedures, further contribute to participant attrition.
Solution
A solution to address these challenges involves the implementation of telemedicine and remote monitoring technologies. By utilising virtual visits, wearable devices, and mobile applications, participants can engage with the trial from the comfort of their homes, reducing the need for frequent clinic visits. This not only enhances convenience for participants but also streamlines data collection, fostering a more participant-friendly and efficient clinical trial environment.
Safety & Side Effect Concerns 18%
The second-highest response to the poll indicated that safety and concerns about side effects are the primary challenges in retaining participants in clinical trials.
Safety and concerns about side effects stand out as a primary challenge in clinical trial participant retention due to their profound impact on participant trust and commitment.
Challenge
Participants may become apprehensive about potential risks associated with experimental treatments, leading to heightened anxiety and a reluctance to continue their involvement in the trial.
Solution
To address this challenge, a digital solution involves the implementation of personalised and real-time communication platforms. Utilizing mobile apps or secure messaging systems, researchers can provide participants with timely and transparent information about safety profiles, potential side effects, and ongoing monitoring. This not only fosters a sense of transparency but also empowers participants to make informed decisions, thereby enhancing their confidence in the trial process and ultimately improving retention rates.
Perceived lack of efficacy 15%
The third-highest response to the poll indicated that the perceived lack of efficacy is the primary challenge in retaining participants in clinical trials.
The perceived lack of efficacy represents a significant challenge in clinical trial participant retention as it directly influences participants’ motivation to continue their involvement
Challenge
If participants perceive that the experimental treatment is not providing tangible benefits or addressing their health concerns, they may be inclined to withdraw from the trial prematurely.
Solution
To counter this challenge, one solution involves implementing personalised and interactive platforms that facilitate ongoing participant engagement. Utilizing mobile apps or web-based interfaces, researchers can provide participants with regular updates on the trial’s progress, individualised feedback on their health metrics, and educational content about the potential benefits of the experimental intervention. This digital approach enhances communication, fosters a sense of involvement, and helps participants understand the potential efficacy of the treatment, ultimately contributing to improved retention rates in clinical trials.
Personal Health Issues 4%
Four percent of respondents elected personal health issues as the primary challenge in retaining participants in clinical trials.
Personal health issues pose a significant challenge in clinical trial participant retention as individuals may grapple with their own health concerns, making it difficult to commit to the demands of a trial.
Challenge
Managing personal health issues can lead to scheduling conflicts, increased stress, and a diminished capacity to fulfil study requirements, ultimately contributing to participant attrition.
Solution
A solution to address this challenge involves the integration of tele-health services and virtual support networks. By incorporating remote monitoring tools and telemedicine platforms, participants can receive personalised support and medical guidance from the comfort of their homes.
This not only mitigates the burden of physical clinic visits but also ensures that participants feel supported in managing their health concerns while actively participating in the clinical trial, thereby promoting better retention rates.
Conclusion
In conclusion, digital solutions emerge as a pivotal strategy to overcome the multifaceted challenges associated with participant retention in clinical trials.
The integration of tele-health, remote monitoring technologies, and virtual communication platforms addresses logistical, practical, safety, efficacy, and personal health issues that often hinder sustained participant engagement.
These innovations enhance convenience, transparency, and personalised support for participants, fostering a more participant-centric and efficient trial environment.
By leveraging technology to bridge the gaps in communication, monitoring, and accessibility, researchers can not only alleviate participant concerns but also streamline trial processes.
Ultimately, the adoption of digital solutions holds great promise in optimising participant experiences, improving adherence to study protocols, and contributing to the success of clinical trials.



