Introduction
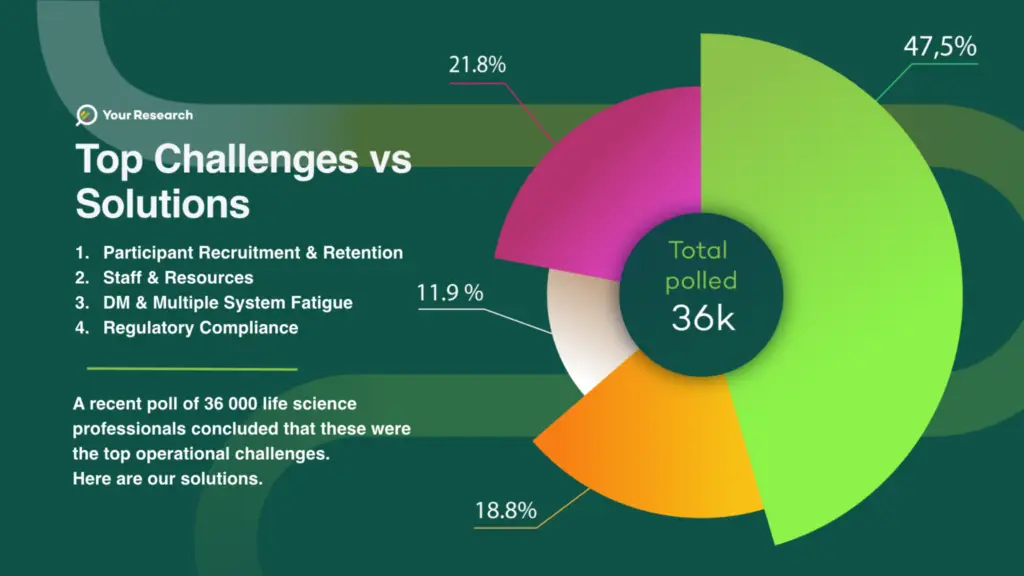
Clinical trials remains the backbone of medical progress, helping to bring innovative therapies and treatments to patients. However, conducting these trials is no small feat, with numerous challenges that can impede progress. Recently, a poll involving 36,000 life science professionals was conducted to uncover the most pressing issues in clinical trial operations. The results were both enlightening and indicative of the industry’s need for digital solutions.
In the forthcoming article, I will delve into the challenge at hand and present a digital solution or tool that could aid in resolving it. While I fully recognise that these challenges require solutions far more intricate than simply incorporating digital technology, I believe it is crucial to emphasise the available resources that can support researchers in this endeavour.
Challenges
Participant Recruitment and Retention: A Pioneering Predicament
A staggering 48% of the professionals surveyed pointed to participant recruitment and retention as the most substantial challenge in clinical trial operations. This challenge underscores the paramount importance of enrolling and retaining the right participants to ensure the efficacy and safety of experimental treatments. A Solution: Digital Patient Recruitment Platforms Digital platforms that harness the power of data analytics, artificial intelligence, and targeted outreach are emerging as a game-changer in the industry. These platforms can identify potential participants more efficiently and personalise communication to engage and retain them throughout the trial.
A Solution: Digital Patient Recruitment Platforms
Recruiting and retaining patients in clinical trials can be a daunting task. Delays in patient recruitment can extend the timeline of a study, increasing costs and potentially affecting the trial’s success. Retaining patients throughout the duration of a trial is equally challenging, as patients may drop out for various reasons, further complicating data collection and analysis.
Staff and Resources: The Hidden Hurdle
For 22% of respondents, the scarcity of staff and resources was the top challenge. As clinical trials become more complex and demanding, the need for qualified personnel and adequate resources is escalating.
A Solution: AI-Driven / Smart Intelligence Systems with Resource Allocation with guided workflow support smart intelligence systems
Leveraging Artificial Intelligence (AI) can help optimise resource allocation and staffing by predicting the necessary human resources and equipment for a given trial. This not only maximises efficiency but also minimises operational costs.
Data Management and Multiple System Fatigue: A Juggling Act
19% of respondents voiced concerns over data management and the phenomenon known as “multiple system fatigue.” Clinical trials involve a myriad of data sources, often managed through separate systems, causing inefficiencies, errors, and delays.
A Solution: Unified Clinical Data Management Systems
Streamlining data management with unified, cloud-based systems can alleviate this issue. These systems offer a holistic approach to data collection, storage, and analysis, reducing the need for manual data entry and integration with other systems, while ensuring data integrity.
Regulatory Compliance: The Ever-Present Challenge
Finally, regulatory compliance was identified as the primary challenge for 12% of those polled. Navigating the complex and ever-evolving landscape of clinical trial regulations is a constant concern for professionals in the field.
A Solution: Regulatory Technology (RegTech)
The advent of Regulatory Technology, or RegTech, is aiding clinical trial teams in ensuring compliance with evolving regulations. Utilising AI and machine learning, these digital solutions can automatically monitor regulatory changes and flag potential non-compliance issues in real time.
Conclusion
In conclusion, the recent poll of 36,000 life science professionals has shed light on the significant challenges faced in clinical trial operations. While recruitment and retention, resource constraints, data management, and regulatory compliance present formidable hurdles, the digital solutions available offer a promising path forward. Embracing these technological advancements will not only make clinical trials more efficient and cost-effective but also accelerate the development of life-saving treatments for patients in need. The industry must seize the opportunities presented by digital innovation to ensure a brighter and healthier future for all.



