Overview
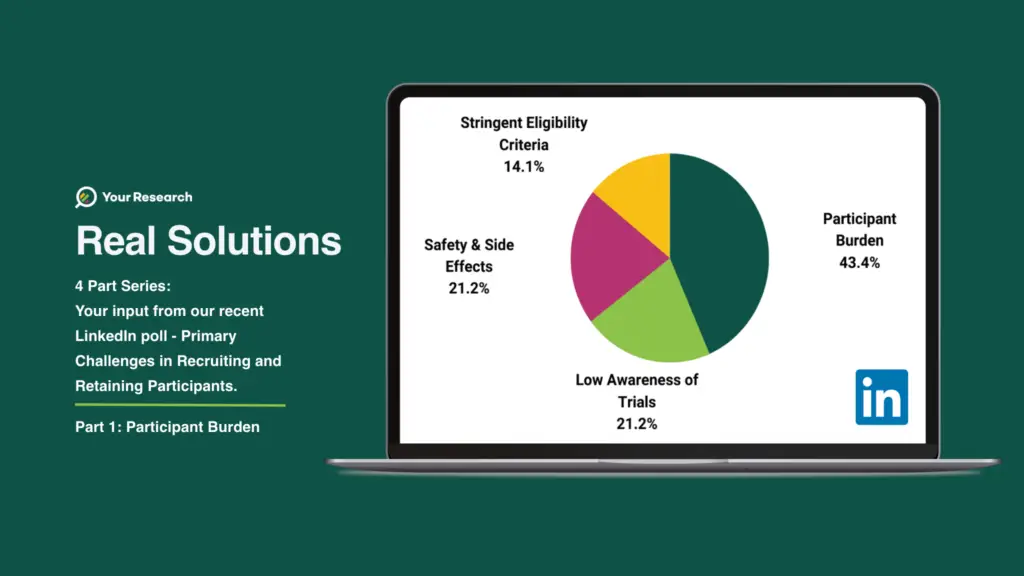
We recently polled your thoughts on our LinkedIn page and the results are displayed in the graph.
Over the coming weeks, we will offer you our insight gained from experience in resolving these 4 challenges, based on our extensive interaction and experience solving them with clinicians and researchers.
The Challenge
The challenge of participant burden in clinical trials refers to the difficulties faced by participants during the trial process. Clinical trials often require participants to undergo various procedures, such as frequent visits to medical facilities, multiple tests, and data collection activities. This burden can lead to non-compliance, dropouts, and inaccurate data, impacting the trial’s validity and success.
The Solution
To address participant burden, software companies like us offer digital solutions to research organizations that streamline and enhance the trial experience for researchers and participants, essentially reducing repetitive tasks through automation and in some cases using Artificial Intelligence (AI).
We also understand that digital technology and AI are not the one and only solution to all research issues, but we have seen them used to improve the current challenges of our clients and partners.
Here are our top 7 fixes for Participant Burden:
1. Remote Data Collection:
Implementing digital tools and wearable devices to remotely collect relevant health data, reducing the need for participants to visit the trial site frequently is key. In essence, use the right digital tools to reduce tedious repetitive or time-consuming tasks. This has dramatic effect on the retention of participants.
2. Mobile Apps and Telemedicine:
User-friendly mobile applications allow participants to communicate with trial coordinators, receive reminders, and access resources. Telemedicine platforms can also enable remote consultations, reducing the need for physical visits. Less time in transit equates to a more efficient participant experience.
3. eConsent and Virtual Onboarding:
Electronic consent processes and virtual onboarding simplify enrolment and reduce the time and effort required from participants. Studies on eConsent versus traditional consent have shown an increase in participant understanding when using multimedia and separating the process. I.e., inform, confirm knowledge, then consent as opposed to all at once.
4. Electronic Diaries:
Digital diaries record symptoms, medication adherence, and other relevant data, making data collection more convenient for participants. Pop-up reminders and prompts also improve the adherence of these participants.
5. Virtual Trial Platforms:
Fully or partially decentralised clinical trials can leverage telemedicine and remote monitoring, minimising the burden of in-person visits. Again, less time in transit, less time burden to participant and less cost to sponsor.
6. Patient Engagement Tools:
Interactive and personalised engagement tools keep participants motivated and informed throughout the trial duration. Gamification of certain tedious tasks can be implemented.
7. Real-Time Monitoring:
Using connected devices and real-time data analytics to identify issues early on, enabling timely interventions and reducing participant burden. Prevent non-adherence by preempting deviations. A good example is using real time monitoring to ensure IP uptake during specific time windows, prompts and reminders for participants and notifications and warning flags sent to researchers to if required intervene.
Case Study
The recent example of the use of participant burden reducing digital software is the “Protea study”

This study benefited from these top fixes by using an electronic questionnaire (ePRO) to gather weekly feedback from parents of 500 babies participating in the year long study.
Conclusion
By implementing these digital solutions, you can enhance your participant engagement, improve data quality, and ultimately ease the burden on participants.



