Reliable service; adaptable to your needs
Interactive Reponse Technology
With the support of the world’s most proven and adaptable service by Almac Clinical Technologies IXRS®3, we offer everything from RTSM to a full suite IRT solution, combined with an exceptional user experience.
At the heart of successful clinical trials, our Interactive Response Technology (IRT) plays a critical role in mitigating risks and ensuring trial integrity.
It manages and automates key processes such as randomisation, drug supply management, and patient enrolment, providing real-time tracking and allocation of study treatments.
This optimises accuracy and efficiency while streamlining logistics, improving compliance, and maintaining the blind, all of which are essential for trial success.
We provide the world’s most reliable IRT service, offering everything from RTSM to a full IRT suite, tailored to clinical trial needs.
Our IRT automates key processes like randomisation, drug supply, and patient enrolment with real-time tracking.
Our IRT streamlines logistics, improves compliance, and maintains blinding for accurate, efficient trial operations.

Key features
Powering trials
Our advanced IRT solution, IXRS 3TM, comes equipped with essential features designed to ensure the success of your clinical trials. Each function is crafted to streamline operations, reduce risks, and enhance efficiency throughout the trial lifecycle.
Automates the randomization process, ensuring patients are allocated to treatment groups with precision and balance, reducing the risk of bias and enhancing trial reliability.
Efficiently manages medication inventory, tracking supply levels in real-time, optimising drug distribution, and minimising waste to ensure treatments are always available when needed.
Simplifies and speeds up the enrolment process, automatically tracking eligibility and onboarding participants, reducing administrative workload and accelerating trial timelines.
Ensures the integrity of blinding protocols, protecting the study from bias, while maintaining full compliance with regulatory requirements to safeguard the trial's validity.
Provides up-to-the-minute visibility on trial progress, including patient status and treatment allocation, enabling faster decision-making and adjustments to enhance trial outcomes.
Automates key logistical tasks such as visit scheduling and supply delivery, reducing delays and ensuring the efficient operation of the trial, from start to finish.
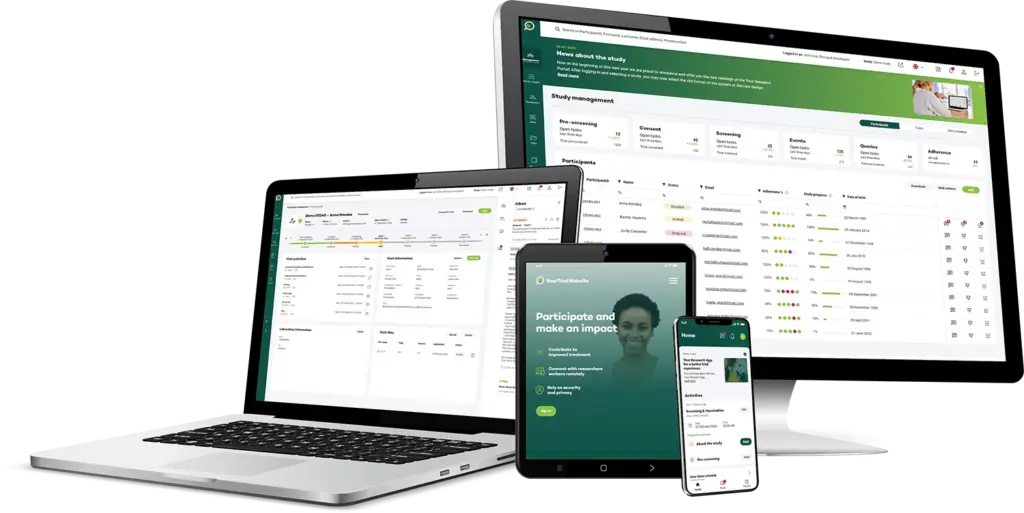
Other Modules to Connect
Your Research provides a range of modules beyond IRT, including CTMS, ePRO, eCOA, and patient engagement tools. These integrate seamlessly to create a unified platform, streamlining trial management, enhancing data accuracy, and improving participant engagement and retention.
Our services
Streamline service delivery to minimize risks and expedite startup
Minimising startup and delay risks is the goal of our services. Whether you prefer a hybrid or full self-service model, we are ready to assist with our expertise and experience, ensuring peace of mind.
Pre-validated questionnaires will save you time setting up your trial and data quality risks
Our network of translators will support with validated translation of questionnaires and communication information for participants.
Together with our technology, local partners and pre-defined documentation, we support you to have oversight into your IRB approval process.
Your Research provides pre-configured devices to patients for consistent performance and controlled study environments based on a hybrid or full provisioning model.
We provide 24/7 assistance to address technical issues and user inquiries, as well as training for clinical staff to ensure they are proficient in using Your Research.