Introduction
The Capish study is set to go live soon! This initiative, spearheaded by Maastricht University and under the guidance of Irene Weltens, employs the innovative Experience Sampling Method (ESM) to gather real-time insights into how nurses, through their behaviour and emotions, contribute to the emergence, prevention, and management of aggressive incidents among patients.
“In close collaboration with the team at Your Research, we have been able to compile the necessary questionnaires in the app to hopefully gain more insight into the daily dynamics of a High Intensive Care unit in psychiatry. Through this study, we hope to contribute to safer care for both staff and patients,” says Irene Weltens.

The Objective?
To explore interactions in the workplace to understand how they affect nurses’ well-being, both at work and at home. Workplace safety and emotional well-being are at the heart of this study, aiming to create a safer and more supportive environment for both healthcare providers and patients.
How does it Work?
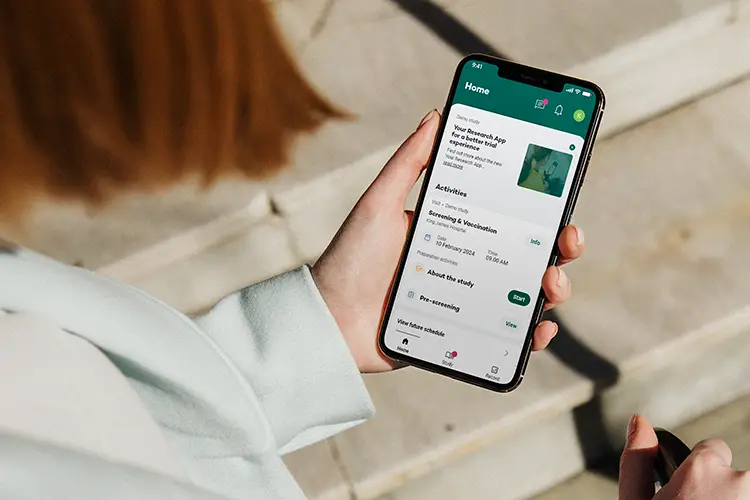
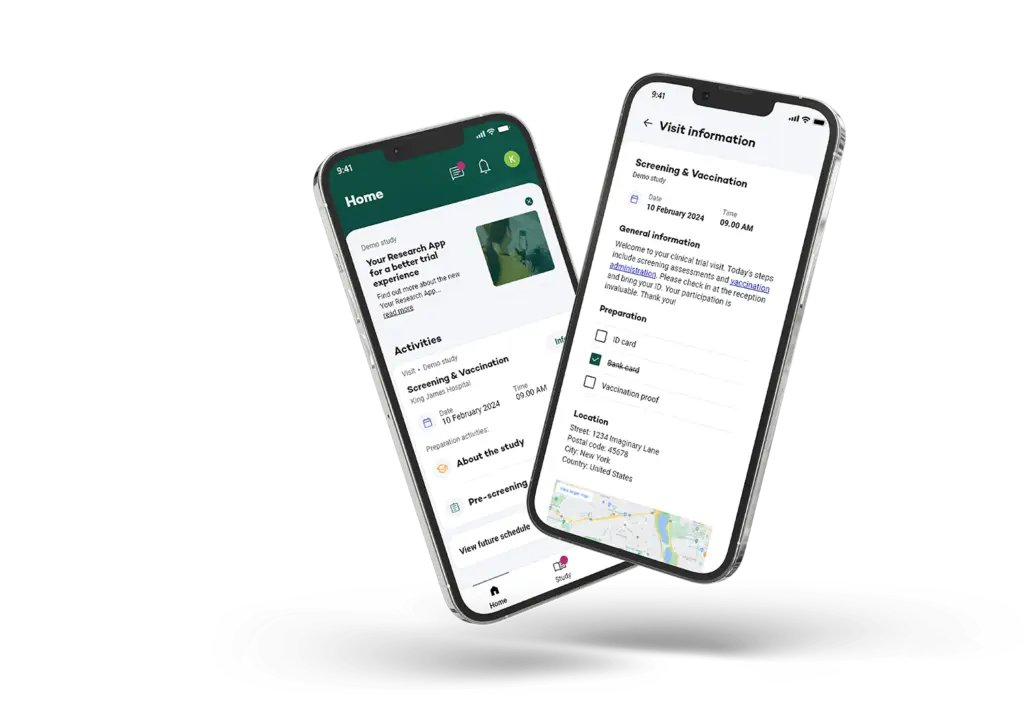
Participating nurses will use the Your Research app, which will send notifications at random times throughout a week. These “beeps” prompt the completion of a short questionnaire about their current experiences and emotions. This real-time data collection, both on and off the job, provides invaluable insights into the complex dynamics of healthcare.