Experience excellent workflow support with prospective insights
Clinical Trial Management System
Your Research’s CTMS is an intuitive, all-in-one solution that streamlines clinical trial management and workflow for sponsors, CROs, and research sites. It provides real-time access to key data and integrates seamlessly with other eClinical tools, enhancing transparency, efficiency, and compliance. The CTMS simplifies processes, ensures data accuracy, and accelerates decision-making for more precise, faster trials.
Facilitates every stage of the trial, from planning and budgeting to monitoring and reporting, ensuring seamless workflows.
Fully compliant with GCP, GDPR, and 21 CFR Part 11 regulations, prioritising the integrity and security of clinical data.
Interface Designed for ease of use, allowing clinical teams to access, update, and review data effortlessly, reducing administrative burden.
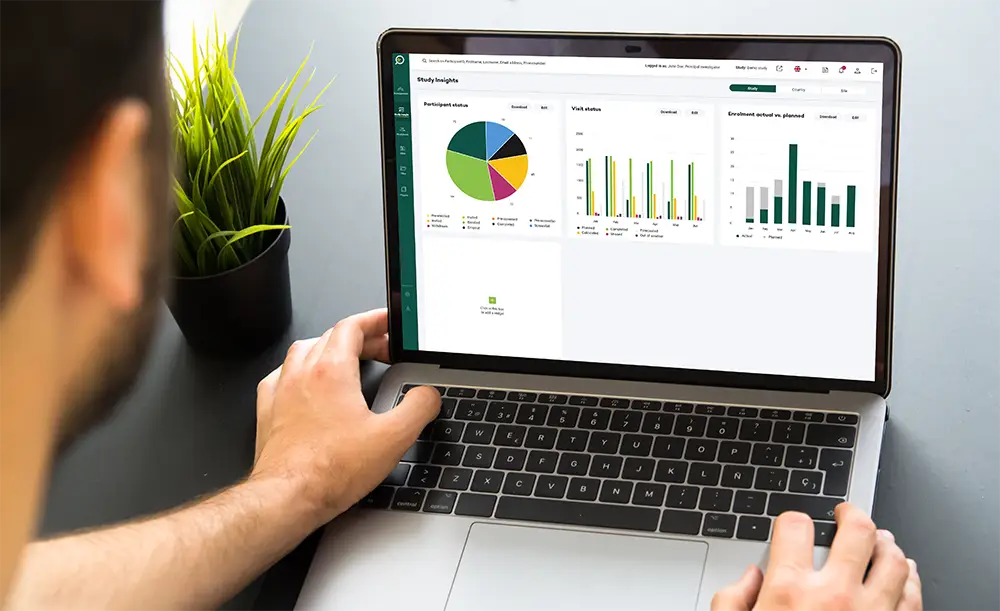
Key features
Unparalleled operational efficiency
Our CTMS is designed to streamline clinical trials by automating workflows, enhancing data-driven decision-making, and providing seamless integration across essential processes.
Discover how our soltuion supports every stage of your trial while reducing complexity and administrative burden.
Fully integrated with the Participant Journey, ensuring smooth coordination and engagement throughout the trial.
Real-time tracking of trial milestones, task assignments, and critical deadlines to maintain study timelines.
In-depth financial tools for cost forecasting, budget tracking, and resource allocation, helping to manage study expenses effectively.
Customisable dashboards and reporting features for monitoring trial metrics, generating insights, and informing data-driven decisions.
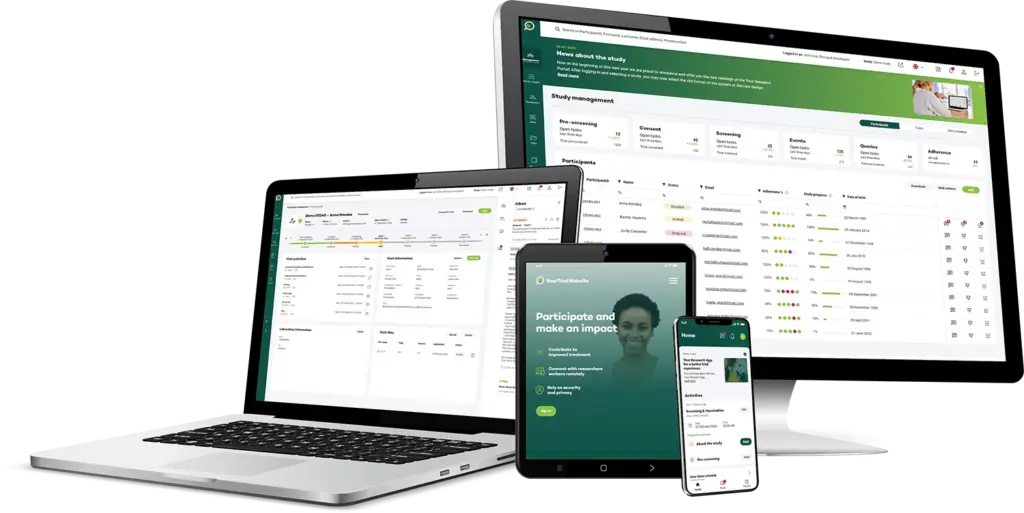
Connected core functionalities
Based on Your Research’s core technology for managing clinical trial workflows, the CTMS provides a range of essential functionalities.
Our services
Effortless trial and technology management with our support
Leveraging Your Research’s modern, user-friendly technology, the CTMS delivers essential functionalities and exceptional services to streamline daily workflows and provide quick responses to queries throughout the day.
Depending on your organisations needs we offer basic to premium support models.
Our in-house support desk operates 24 hours per day and 7 days per week and is globally accessible.
In our extensive knowlegde base we have articles and video's available for the most frequently asked questions.
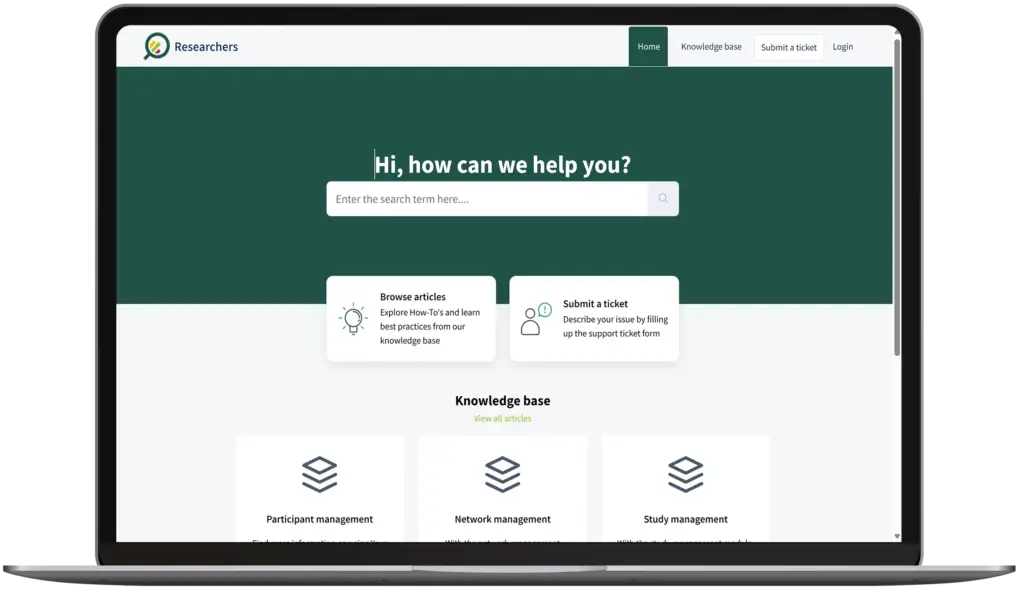
Modules at a glance
Elevate the whole Participant Journey
Serving as the essential foundation, our Unified Protocol enhances trial outcomes and facilitates the seamless integration of both modules and third-party systems along the Participant Journey.
This foundational structure ensures comprehensive oversight and enables strategic foresight throughout the patient journey.





