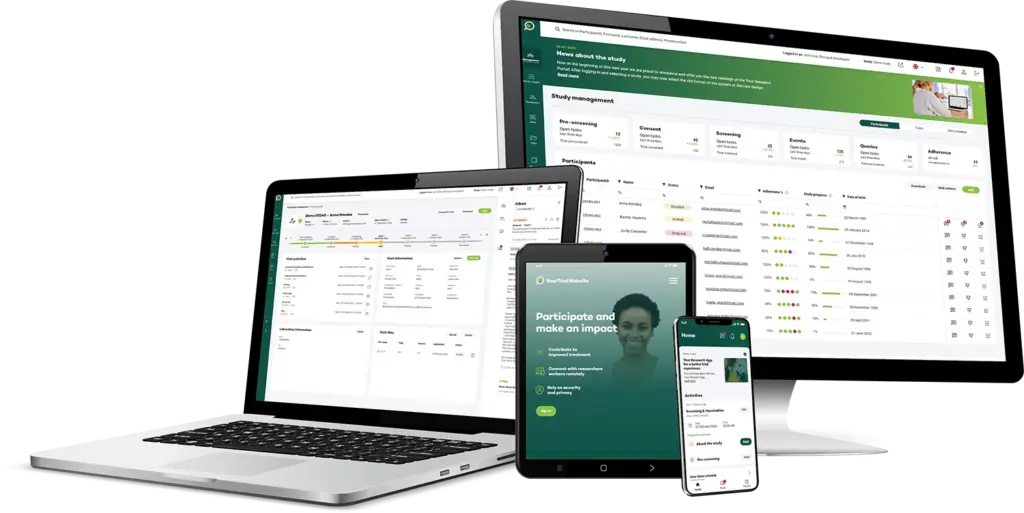
Streamlined Participant Interaction and Data Management
Clinical Trial Research App
Discover the comprehensive Research Clinical Trial app, designed to revolutionize participant engagement and data management. Available as both a web and native application, this tool empowers participants by providing easy access to their contact details and enabling seamless communication with the study team or site through chat or video calls. Participants can collect data using ePRO, eDiaries, and connected devices, view and optionally manage their data, and even use the app offline. The app is adaptable to study or organizational branding, enhancing participant experience with proven usability tested by over 150,000 users. Notifications ensure participants stay informed, keeping them engaged and on track throughout the trial.
Empower participants with the ability to easily access contact information and communicate with the study team via chat or video calls, fostering better engagement and trust throughout the trial.
Enable participants to gather data through ePRO, eDiaries, and connected devices while having the option to view and manage their collected data. This comprehensive approach ensures accurate and efficient data collection, even offline.
Benefit from an app tested by over 150,000 participants for optimal ease of use, which can be customized to reflect the branding of your study or organization for a cohesive user experience.
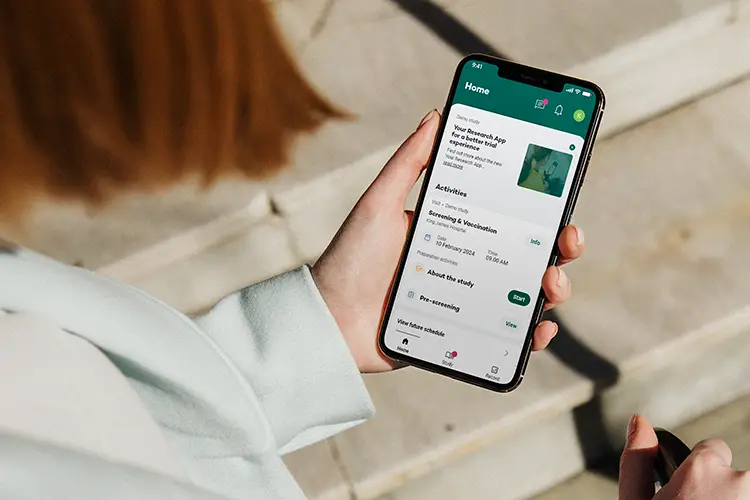
Key features Participants
Engage Participants
Our unique Visit Management module makes it easy to schedule visits according to protocol requirements, with a built-in forecasting tool that alerts you when an upcoming action may be missed.
Supporting both automated and manual scheduling based on visit type, this module is directly linked to available resources for seamless coordination across all visit types, including participant, investigator, and monitor visits.
Facilitate data gathering through ePRO (electronic patient-reported outcomes) and eDiaries, enhancing the accuracy and real-time capture of participant data.
View, modify, and provide consent using globally compliant electronic signatures.
Participants can view upcoming visits, including location details and preparation instructions. This feature, combined with chat and video call options, allows participants to easily reach out for questions or clarifications with the study team.
Initial login with 2FA, followed by simple PIN or biometric access for ongoing sessions.
Keep participants informed and engaged with reminders and updates throughout the study process.
Participants can not only view but also manage their collected data, providing them with greater transparency and control.
Optional chat and video call functions to strengthen participant-study team communication securely.
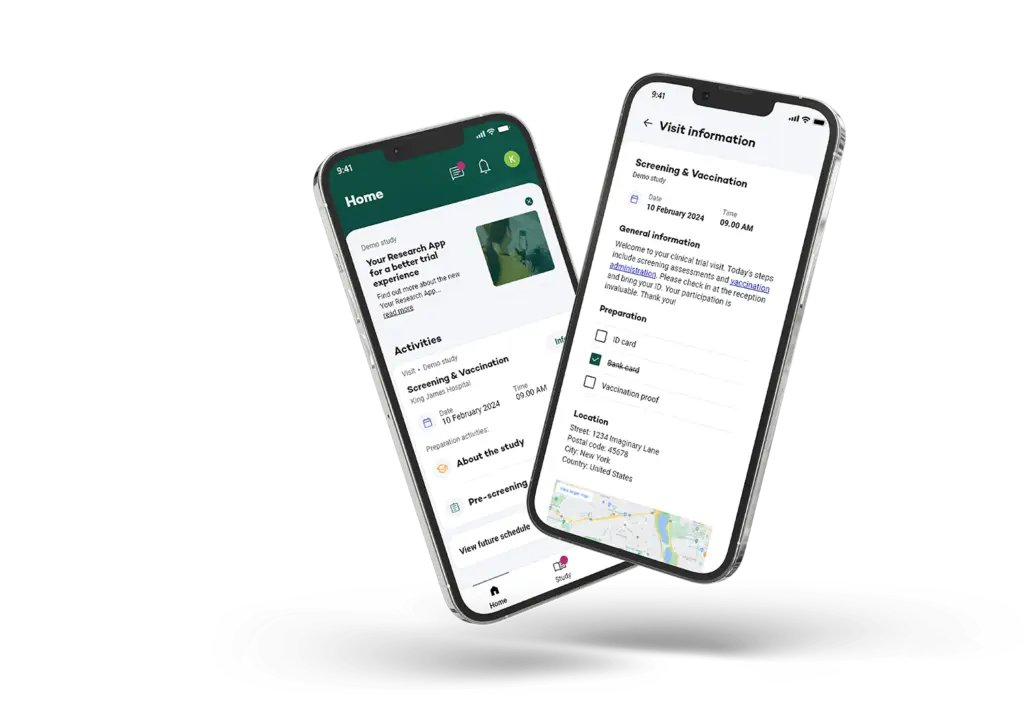
Customizable Modules for Your Clinical Trial
The Research Clinical Trial app includes key modules such as ePRO and eDiaries, consent management, visit scheduling, real-time communication, event detection, and notifications. The app can be tailored with these modules to fit the unique needs of your study, ensuring an efficient and engaging participant experience.
Key Benefits
Improve protocol adherence and efficiency
Ensure efficient coordination, reduce site administrative burden, and enhance participant compliance. Making the management of clinical trials more streamlined and effective, resulting in high-quality data.
Study teams can monitor participant engagement and progress, ensuring timely follow-ups and data accuracy.
Keep participants informed and engaged with reminders and updates throughout the study process.
Ensure participants can continue data collection and interaction without interruption, even when not connected to the internet.
Study teams can view data real-time and process changes based on a protocol including a comprehensive audit trail.
Integrated event detection functionality allows study teams to be automatically alerted to critical data points or deviations, enabling timely intervention.