Introduction
In clinical research, the selection of a Clinical Trial Management System (CTMS) plays a pivotal role in ensuring the efficiency and success of clinical trials. With technology constantly evolving, 2024 brings forth a new set of considerations for researchers looking to adopt or upgrade their CTMS.
In this article, we will explore best practices, security and compliance, as well as crucial factors we polled life science professionals to determine what is the top consideration for selecting a CTMS.
The poll asked the question:
How did you or when you plan to, choose a Clinical Trial Management system (CTMS)? Select your top metric in the order of priority:
A. Value for money
B. Interoperability
C. Usability
D. Customisability
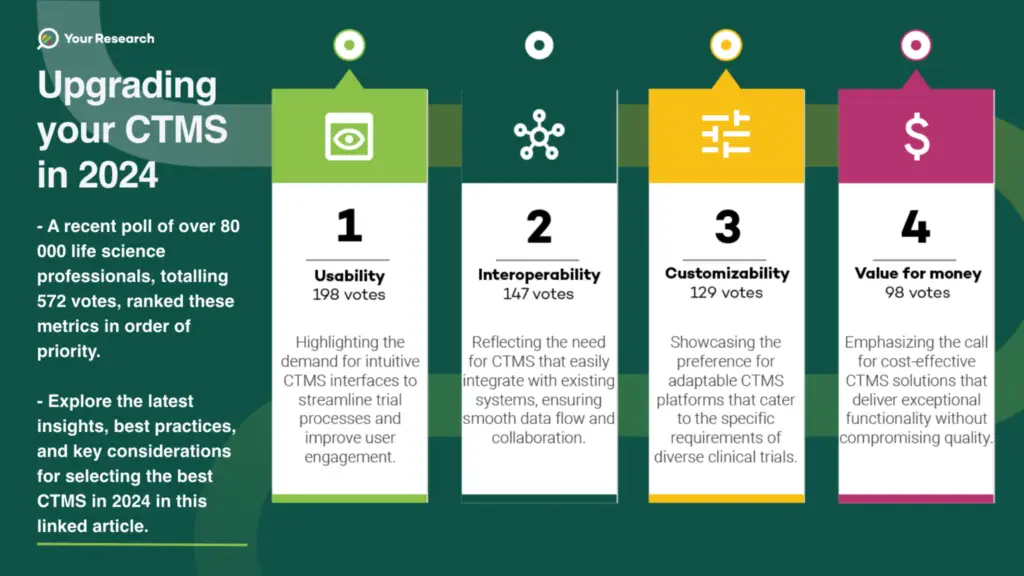
This question was presented to a group of 560 785 Life Science Professionals, among whom 83 087 had the opportunity to respond during the week the poll was available. Of those, 572 completed the poll.
The results are expressed below in percentages.
Best Practices to look out for
When it comes to choosing a CTMS, best practices encompass a range of factors.
Start by evaluating the system’s ability to streamline trial processes, manage data effectively, and support collaboration among the research team. Opt for a system that integrates seamlessly with existing workflows, reducing the learning curve for users.
Additionally, a user-friendly interface and comprehensive training resources contribute to a smoother onboarding process, enhancing overall productivity.
Security and Compliance: critical for FDA and EMA inspection
Security and compliance are non-negotiable aspects of any CTMS. Ensure that the system adheres to the latest regulatory requirements and industry standards. Robust data encryption, audit trails, and access controls are paramount to safeguard sensitive patient information. Verify that the CTMS provider undergoes regular security audits and stays abreast of emerging threats to maintain a secure environment for trial data.
Standards to expect your CTMS to be compliant with should include:
1. HIPAA (Health Insurance Portability and Accountability Act):
Ensures the protection of patients’ health information.
Establishes standards for the privacy and security of electronic health records (EHR).
2. 21 CFR Part 11:
Focuses on electronic records and electronic signatures in the context of clinical trials.
Ensures the reliability and trustworthiness of electronic documentation and signatures.
3. Good Clinical Practice (GCP):
An international standard for the design, conduct, performance, monitoring, auditing, recording, analysis, and reporting of clinical trials.
Emphasises data integrity, patient safety, and regulatory compliance.
4. ISO 27001:
An international standard for information security management.
Requires the implementation of a systematic approach to managing sensitive company information.
5. EU General Data Protection Regulation (GDPR):
Protects the privacy and personal data of European Union citizens.
Enforces strict guidelines on the processing and handling of personal data, including data related to clinical trials.
6. FDA 21 CFR Part 50:
Outlines the protection of human subjects involved in clinical trials.
Emphasises informed consent, documentation, and ethical treatment of participants.
7. FDA 21 CFR Part 54:
Focuses on financial disclosure by clinical investigators.
Ensures transparency and addresses potential conflicts of interest in clinical trials.
8. ICH E6 (R2):
An international standard for the conduct of clinical trials.
Emphasises risk-based approaches to clinical trial design, conduct, monitoring, and reporting.
Points to consider from the poll:
1. Usability 34.6%
User-friendly interfaces simplifying ease of use and adoption.
User experience is a critical factor in the successful adoption of a CTMS. An intuitive interface, coupled with features designed to simplify complex tasks, promotes user engagement. Prioritise a system that aligns with the diverse skill sets of your research team, minimising the learning curve and optimising daily operations.
2. Interoperability 25.6%
Seamless integration with existing tools, facilitating process automation, and secure data migration between platforms.
In a connected world, interoperability is key. A CTMS that seamlessly integrates with other clinical systems, electronic health records, and external databases fosters a cohesive research environment. This not only enhances data accuracy and consistency but also facilitates collaboration with external partners, enabling a more comprehensive view of the trial ecosystem.
3. Customisability 22.5%
High level of customisation, particularly in reports and analytics and process to fit diverse procedures, policies, and practices within organisations, ensuring adaptability.
Every clinical trial is unique, and a one-size-fits-all approach may not be suitable. Look for a CTMS that offers a high degree of customisability, allowing you to tailor the system to your specific trial requirements. From data fields to workflow configurations, flexibility ensures that the CTMS aligns seamlessly with the nuances of your research.
4. Value for money 17.1 %
Assessing cost-effectiveness, aligning features with organisational needs.
In the realm of clinical trials, budgets are often constrained. It’s crucial to assess the value a CTMS brings to your organisation in relation to its cost. Consider the features provided, scalability, and potential for future upgrades. Striking the right balance between functionality and affordability ensures a cost-effective solution without compromising on essential capabilities.
Conclusion
Not all CTMS systems are created equal. Regular assessments are paramount to ensure that your chosen system evolves with industry standards and technological advancements.
By prioritising best practices, security, and the key factors of interoperability, usability, customisability, and value for money, researchers can navigate the complex and dynamic landscape of clinical trial management with confidence.
Top tip: Stay proactive in evaluating and upgrading your CTMS to uphold the highest standards of efficiency and data integrity in your clinical trials.



