Introduction
Recruiting participants for clinical research is a critical aspect of advancing medical knowledge and improving patient outcomes. However, the process is riddled with challenges that often hinder the progress of research studies.
In a recent poll conducted among more than 40,000 life science professionals, several key issues emerged, shedding light on the difficulties faced in participant recruitment.
This article explores four major challenges — Limited Awareness, Eligibility Criteria, Patient Mistrust, and Healthcare Worker Engagement — and delves into potential solutions to overcome these obstacles.
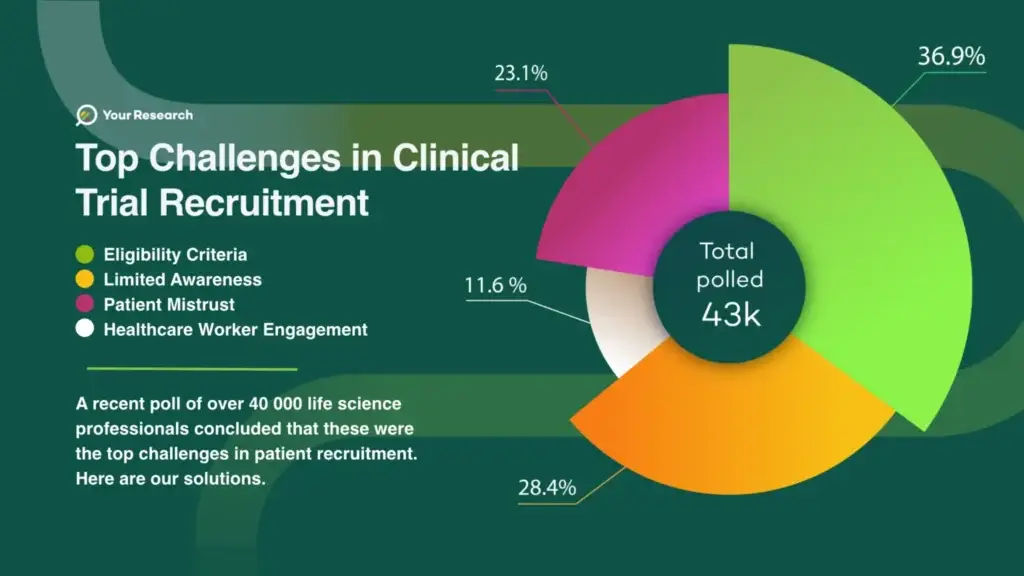
Eligibility Criteria 36.9%
One of the primary challenges in the successful execution of clinical trials is the recruitment of eligible participants.
Eligibility criteria serve as essential guidelines to ensure the safety and efficacy of the trial, but they also pose significant hurdles in finding suitable candidates.
Challenge: Complexity and Stringency of Eligibility Criteria
Eligibility criteria for clinical trials are often complex and stringent, designed to identify a specific patient population for the study. However, this complexity can lead to difficulties in finding eligible participants, resulting in prolonged recruitment timelines. Patients may be excluded based on various factors, such as co-morbidities, age, or specific medical histories.
Solution: Utilise Natural Language Processing (NLP) Algorithms
Implementing NLP algorithms can streamline the screening process by efficiently analysing electronic health records (EHRs) and patient data. NLP can quickly identify individuals who meet the eligibility criteria, minimising the manual effort required for screening. This not only accelerates the recruitment process but also ensures a more comprehensive evaluation of potential participants.
Limited Awareness 28.4%
Many individuals are unaware of ongoing research studies, and even if they do have some knowledge, the details are often unclear or misunderstood.
Solutions such as targeted social media campaigns and interactive websites, can play a pivotal role in disseminating information about clinical trials. Utilising platforms where potential participants frequently engage can enhance awareness and provide easily digestible information about the importance and benefits of participating in research.
Challenge: Limited Awareness and Access to Clinical Trials
Many individuals who may be eligible for clinical trials are unaware of their existence or lack access to information about ongoing studies. This lack of awareness contributes to a limited pool of potential participants, making it challenging for researchers to find suitable candidates.
Solution: Develop a User-Friendly Clinical Trial Matching Platform
Create a user-friendly digital platform that provides easily accessible information about ongoing clinical trials. This platform can leverage machine learning algorithms to match potential participants with relevant trials based on their health profiles. By enhancing visibility and simplifying the search process, this solution can increase awareness and participation in clinical trials.
Patient Mistrust 23.1%
Patient mistrust in the medical and research community is a persistent issue that hampers recruitment efforts. Historical incidents of unethical conduct and inadequate communication have contributed to this skepticism. Building trust requires transparency and open communication.
Secure online forums and virtual town hall meetings, can provide a platform for researchers to address concerns, share study information, and engage directly with potential participants. Establishing a trustworthy online presence is crucial in fostering a sense of transparency, thereby mitigating patient mistrust.
Challenge: Lack of Transparency, Data Security and Privacy
Patients often express mistrust when there is a lack of clear information and transparency about the clinical trial process, potential risks, and benefits. Uncertainty about the purpose and procedures of the study can lead to skepticism and reluctance to participate.
Patients may hesitate to participate in clinical trials due to concerns about the security and privacy of their personal information. The fear of data breaches or misuse can lead to a reluctance to share sensitive health data.
Solution: Develop Interactive Educational Platforms and Implement Blockchain Technology for Data Security
Create interactive digital platforms that offer comprehensive information about clinical trials in an easily understandable format. Incorporate multimedia elements, such as videos and infographics, to explain the trial process, potential risks, and benefits. These platforms can empower patients with knowledge, fostering trust through transparency.
Leverage blockchain technology to enhance the security and privacy of patient data in clinical trials. Blockchain ensures that data is encrypted, transparent, and tamper-resistant, providing a secure and trustworthy environment for storing and managing sensitive information. By addressing data security concerns, this digital solution aims to instill confidence in patients regarding the protection of their personal information.
Healthcare Worker Engagement 11.6%
Involving healthcare workers in the recruitment process is crucial, as they are often the primary point of contact for potential participants. However, engaging busy healthcare professionals poses its own set of challenges.
Dedicated online portals with easily accessible study information, regular webinars, and digital training modules can enhance the knowledge and engagement of healthcare professionals. These platforms can serve as a one-stop resource for healthcare workers, enabling them to stay informed about ongoing research studies and effectively communicate study details to potential participants.
Challenge: Inefficient Patient-Physician Communication
Effective communication between patients and healthcare providers is crucial for identifying eligible participants. However, the traditional methods of communication may not be efficient, leading to misunderstandings or missed opportunities for participation.
Solution: Implement Mobile Health (mHealth) Applications
Introduce mHealth applications that facilitate direct communication between patients and healthcare providers. These applications can provide real-time updates on available clinical trials, eligibility criteria, and appointment schedules. Improved communication through mobile platforms enhances patient engagement and ensures that both parties stay informed throughout the recruitment process.
Conclusion
Overcoming the challenges in recruiting participants for clinical research requires a multifaceted approach, and digital solutions play a pivotal role in addressing these issues. By leveraging targeted awareness campaigns, streamlined eligibility screening processes, transparent communication platforms to tackle patient mistrust, and engaging healthcare workers through digital mediums, the clinical research community can enhance participant recruitment efforts.
Embracing these solutions not only improves the efficiency of the recruitment process but also fosters a more inclusive and informed participant pool, ultimately advancing medical research and contributing to the betterment of healthcare for all.



