Introduction
The Protea study, an innovative paediatric trial focusing on prematurely born children in the Netherlands, has introduced eConsent to address challenges in the informed consent process. This digital transformation is a crucial step to enhance the logistics of this specific study and can serve as an example for other trials.
Specific Challenges in the Protea Study
- Growing participant regions: The expansion of participant regions throughout the Netherlands has resulted in an increase in home visits, where prior informed consent is essential.
- Delays due to postal processes: The necessity to send documents by post leads to delays, especially if mail gets lost or if informed consent forms are incorrectly filled out.
- Physical appointments with only one parent: In cases where only one parent accompanies the child to the appointment, it is necessary for the absent parent to sign the form beforehand, a step often overlooked in practice.
- Fully virtual study visits: Situations where all study visits take place via video calls highlight the need for a digital solution.
- Logistics of study medication delivery: During the first study visit, study medication is directly handed over to parents. This requires timely randomization and signing of the informed consent form, ideally before the first study visit.

Why eConsent is the Solution
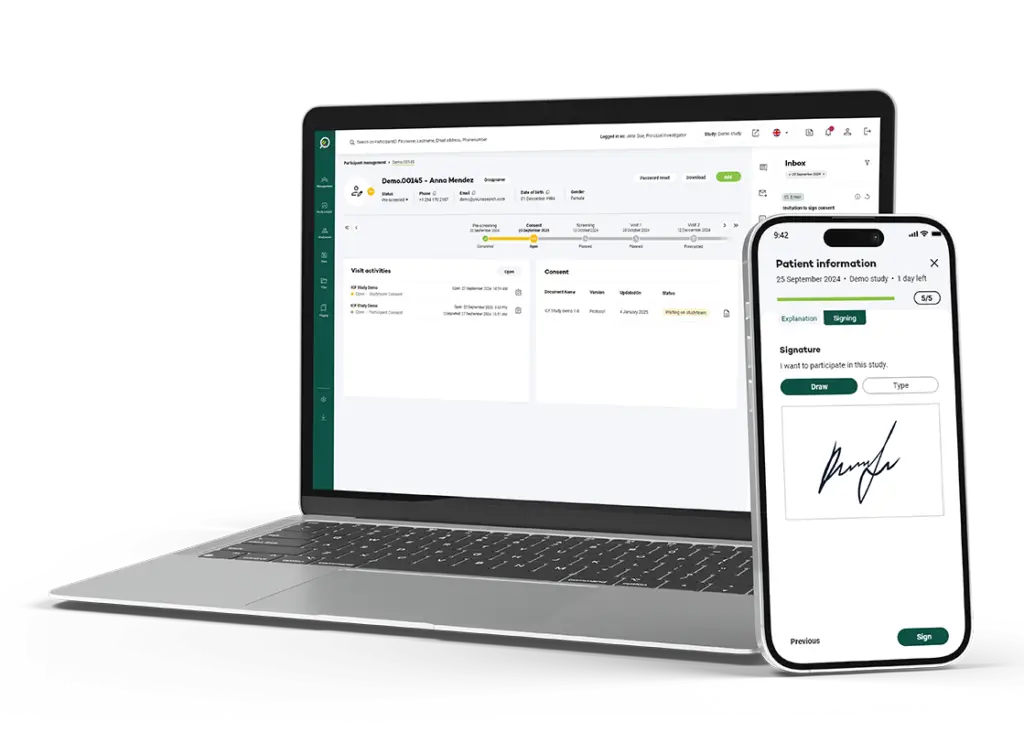
eConsent offers an elegant solution to these challenges, allowing parents to digitally sign the informed consent form, significantly improving the efficiency of the process for both parents and the study team.
- Accessibility: Parents can conveniently digitally sign the consent form, especially useful in situations where all study visits are virtual or when randomization and medication distribution must occur at the first visit. The forms and their choices are always easily accessible in one place.
- Flexibility: Parents preferring paper forms retain this option.
- User-friendliness: The young target audience and their parents have shown interest in the digital option, recognizing the time-saving benefits in their busy lives with young children.
- Efficiency: Digital signing accelerates the informed consent process, crucial for medication trials like Protea, where timely delivery of study medication is essential.
METC Approval
The approval process proceeded smoothly, thanks in part to the experience and expertise of Your Research, the technology provider. The necessity was evident to the METC, and the safety and reliability were well ensured. The fact that the solution had already been approved by several other METCs saved a considerable amount of time and effort.
In addition to eConsent, Your Research had already provided support to the Protea study in data collection and management (via the RespiRecord App and its dashboard) and optimizing other logistical processes in participant communication. The implementation of their technology resulted in a significant reduction in the use of, for example, Excel files. This led to more efficient data management and analysis, making the implementation of eConsent a logical next step. It’s important to note that the applications fully comply with GCP and AVG regulations.
About the Protea Study
Premature birth is common (8-10% of deliveries) and is associated with respiratory issues in the early years, posing a risk factor for respiratory diseases later in life. The Protea study aims to prevent respiratory infections and ‘baby asthma’ in the first years after premature birth through treatment with bacterial lysates. These lysates contain harmless fragments of bacteria that strengthen the immune system. In older children, this treatment has proven effective in preventing respiratory infections. Our study group is currently investigating whether prevention is possible in 500 prematurely born children through treatment in the first year of life. If treatment with bacterial lysates proves to have a favorable effect on respiratory issues after premature birth, we expect a significant impact on the quality of life for many children and parents. For monitoring respiratory issues and other health problems, parents use the RespiRecord App, specifically designed to monitor respiratory issues in children easily. This greatly helps us collect data in a structured and reliable manner.
www.proteastudie.nl



