Efficient, Compliant, and Centralized Document Solutions
Document Management for Clinical Trials (TMF & ISF)
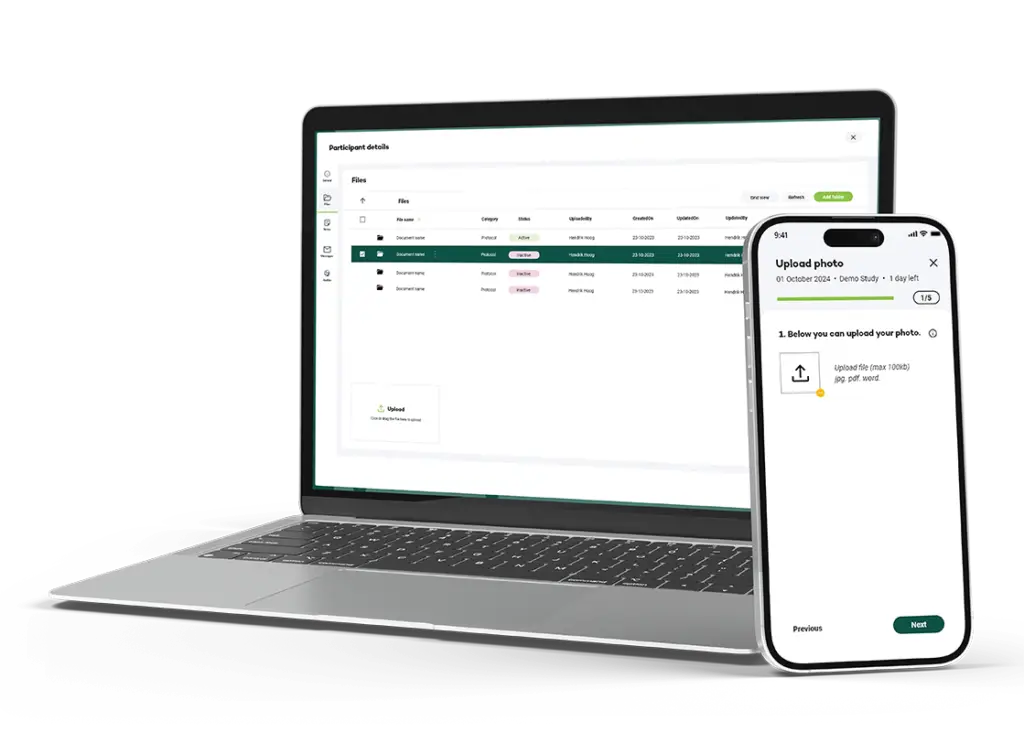
Your Research offers a Document Management module designed specifically for Trial Master File (TMF) and Investigator Site File (ISF) management in clinical trials. Our solution centralizes all essential documents, streamlining compliance, improving accessibility, and enhancing oversight across every stage of the study. With a secure, compliant, and user-friendly platform, your team can focus on trial progress, confident that all documentation requirements are met.
Centralized storage and permission-based access make it easy for authorized users to access and manage documents, saving time and reducing bottlenecks.
Automated tracking, version control, and audit trails ensure that your TMF and ISF are always up-to-date, compliant, and ready for inspections, simplifying the audit process.
Secure, real-time access to documents allows CROs, sponsors, and sites to collaborate effectively, ensuring all necessary documents are available and aligned with regulatory requirements.

Key features
Streamlined Document Management
Your Research’s Document Management module for TMF and ISF is a comprehensive, compliant solution designed to optimize document workflows, improve compliance, and reduce administrative burden. With centralized, secure storage and seamless integration, we provide a reliable foundation for efficient document management in every clinical trial.
Store, organize, and manage all TMF and ISF documents in a single, centralized platform that is accessible to authorized users at any time. This centralized storage ensures all trial documents are up-to-date, well-organized, and easily retrievable.
Maintain the integrity of your documents with automated tracking and version control, allowing your team to easily manage document updates, revisions, and approvals while ensuring that only the latest versions are in use.
Ensure full compliance with ICH-GCP, FDA, EMA, and other regulatory requirements. Our document management solution provides built-in tools to simplify audit preparation and inspection-readiness, so your TMF and ISF remain compliant with global standards.
Control document access at a granular level, enabling secure, role-based permissions to ensure that only authorized team members have access to specific files, enhancing data security and regulatory compliance.
Access pre-configured templates and SOPs for TMF and ISF documentation, ensuring consistent file organization and adherence to trial-specific standards. This helps standardize processes and maintain high-quality documentation across all sites.
The Study Website integrates with the Pre-Consent and Visit Management modules, providing participants with a cohesive journey from initial interest to scheduling and beyond. This integration simplifies coordination for the site and ensures participants remain engaged and informed.
Connect with other modules
Your Research provides a range of modules beyond Document Management, including CTMS, IRT, ePRO, eCOA, and patient engagement tools. These integrate seamlessly to create a unified platform, streamlining trial management, enhancing data accuracy, and improving participant engagement and retention.





