Request Proposal Valuable insights from caregivers and observers to enrich study data
ObsRO
Observer-Reported Outcomes (ObsRO) provide crucial information on participants who may not be able to fully report their own symptoms, such as children, elderly patients, or those with certain health conditions. Your Research’s ObsRO module enables caregivers and observers to document participant behaviors, responses, and well-being accurately, offering trials an additional layer of data that reflects real-world observations and experiences.
ObsRO provides valuable insights into participant behaviors and experiences, especially when self-reporting isn’t possible. Observations from caregivers and family members offer unique data that reflects real-world conditions.
Observer-friendly tools standardize data collection, ensuring that trial data is accurate, consistent, and captures meaningful aspects of participant experiences.
Perspectives
Combined with eCOA, ClinRO, and ePRO, ObsRO data contributes to a multi-dimensional view of treatment impacts, capturing observer, clinician, and participant-reported outcomes.
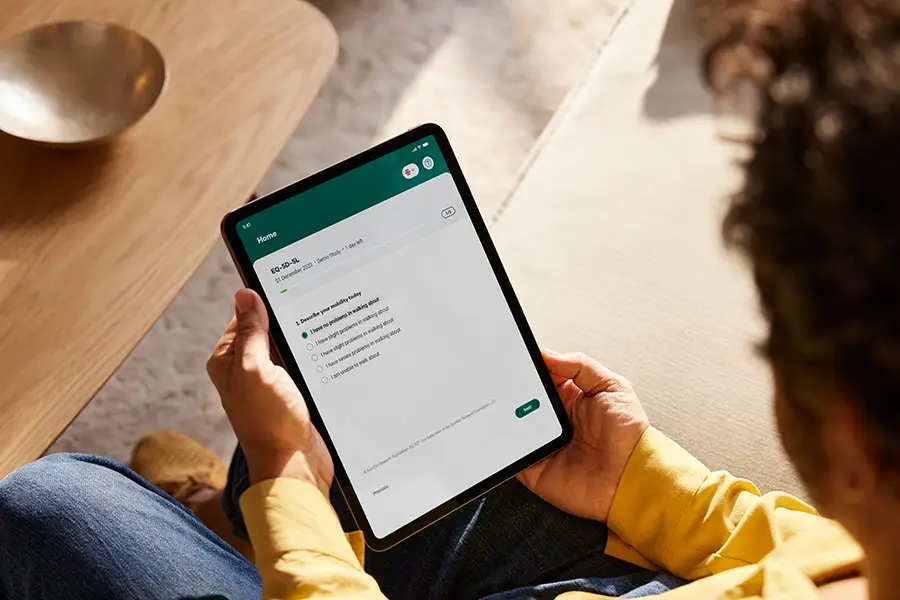
Key features
Enhancing treatment insights
Your Research’s ObsRO module supports trials by collecting meaningful insights from caregivers and other observers, enhancing data quality and broadening the understanding of treatment impacts. Through integration, security, and compliance, we ensure that observer-reported outcomes add valuable, real-world depth to clinical trial data.
Equip caregivers and observers with user-friendly, validated tools and questionnaires, designed to capture consistent data on participant behaviors and experiences. These tools ensure reliable documentation, supporting high-quality insights directly from the observer’s perspective.
Our ObsRO module integrates smoothly with other outcome assessments like eCOA, ClinRO, and ePRO, creating a unified solution for collecting caregiver, clinician, and participant perspectives. This comprehensive view enables richer analyses and more complete data interpretations.
Observers can enter data in real-time, providing up-to-date information on participant well-being and responses. All ObsRO data is securely stored and accessible to authorized users, ensuring data integrity and privacy for participants.
Our ObsRO module is built to meet global regulatory standards, including ICH-GCP, FDA, and EMA requirements. With transparent audit trails and comprehensive logging, all observer-reported data is inspection-ready.
Built-in reminders help observers stay on track with assessments, reducing the risk of missed data collection points and ensuring all observations align with study protocols.
Connect with other modules
Your Research provides a range of modules beyond Document Management, including CTMS, IRT, ePRO, eCOA, and patient engagement tools. These integrate seamlessly to create a unified platform, streamlining trial management, enhancing data accuracy, and improving participant engagement and retention.
