Introduction
Clinical trials are crucial for advancing medical research, testing new treatments, and improving patient care. However, the success of these trials relies heavily on effective engagement and recruitment processes.
In recent weeks, Your Research has conducted polls within the life science industry to determine the top four challenges in research operations. Our article on this can be found here.
A resounding 47.5% of responses highlighted Participant Recruitment & Retention as the top challenge.
In this article, we will delve into the key stages of clinical trial participation—patient identification, recruitment/enrolment, and retention—while shedding light on the challenges faced and proposing possible solutions.
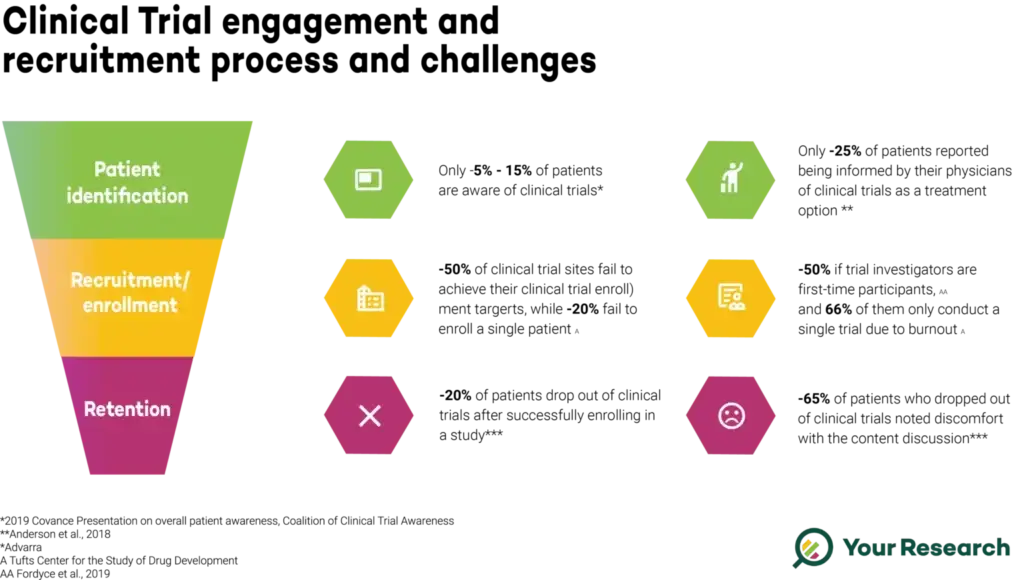
Patient Identification - Bridging the Awareness Gap
Patient identification is a pivotal stage in the clinical trial process, serving as the foundation for successful participation. Despite the immense importance of clinical trials in advancing medical science, a startlingly low percentage—estimated to be between 5-15%—of eligible patients are aware of ongoing trials. This lack of awareness poses a significant barrier to the recruitment of diverse and representative participant populations. Understanding the challenges that contribute to this low awareness is crucial for developing effective solutions.
Challenges in Patient Identification:
Limited Public Awareness
One primary challenge is the limited general awareness of clinical trials among the public. Many individuals are unfamiliar with the existence of clinical trials, their potential benefits, and how participation contributes to medical advancements. This lack of understanding creates a substantial hurdle in identifying eligible candidates.
Fragmented Information Dissemination
Information about clinical trials is often scattered across various sources, making it challenging for potential participants to find relevant and accurate details. This fragmentation of information is exacerbated by the diverse nature of trials, spanning different medical conditions and geographic locations. As a result, eligible individuals may miss out on opportunities simply due to the difficulty of accessing pertinent information.
Stigma and Mistrust
There exists a pervasive stigma surrounding clinical trials, fuelled by concerns about experimental treatments, potential side effects, and mistrust in the healthcare system. Overcoming these barriers requires building trust and dispelling misconceptions. The challenge lies in addressing these concerns effectively to encourage participation and foster a sense of confidence in the trial process.
Solutions to Enhance Patient Identification:
Centralised Trial Information Platforms
Creating centralised online platforms that consolidate information about various clinical trials can significantly improve accessibility. These platforms should be user-friendly, providing clear details about trial objectives, eligibility criteria, and potential benefits. This centralisation helps potential participants navigate the complex landscape of clinical trials more efficiently.
Digital Marketing and Social Media Campaigns
Leveraging digital marketing strategies and social media platforms can enhance outreach efforts. Tailored campaigns can be designed to target specific demographics, raising awareness about clinical trials and addressing common concerns. Engaging content, such as success stories and testimonials, can humanise the trial experience, making it more relatable and less intimidating.
Telehealth and Virtual Engagement
The integration of tele-health services and virtual engagement options can overcome geographical barriers and enhance patient identification. Virtual informational sessions, online consultations, and interactive webinars can facilitate direct communication between potential participants and researchers, addressing queries and building trust in a convenient and accessible manner.
Addressing the challenges in patient identification in clinical trials requires a strategic integration of digital solutions. By enhancing public awareness through centralised platforms, targeted digital campaigns, and virtual engagement options, the clinical research community can bridge the awareness gap, ultimately leading to a more diverse and engaged participant pool. These digital innovations not only streamline the recruitment process but also contribute to a more inclusive and representative landscape in clinical trials.
Recruitment and Enrolment: Overcoming Challenges for Success
Recruitment and enrolment are pivotal phases in the clinical trial process, determining the success and efficiency of the study. Despite the critical importance of these stages, statistics show that only around 50% of clinical trial sites manage to achieve their enrolment targets. This gap between expectations and reality underscores the complexities involved in participant recruitment. Examining the challenges and exploring digital solutions can shed light on ways to enhance these crucial aspects of clinical research.
Challenges in Recruitment and Enrolment:
Participant Hesitancy and Lack of Trust
A significant challenge in recruitment stems from participant hesitancy and a lack of trust in the clinical trial process. Concerns about potential side effects, the experimental nature of treatments, and the perceived risks associated with participation contribute to individuals hesitating to enrol. Building trust becomes paramount, and this challenge requires strategies to address misinformation and enhance transparency.
Stringent Eligibility Criteria
Many clinical trials impose stringent eligibility criteria, narrowing the pool of eligible participants. While these criteria are necessary for the scientific integrity of the study, they can be a major hindrance in achieving enrolment targets. Striking a balance between maintaining scientific rigor and broadening eligibility criteria to include a more diverse population is a delicate challenge that researchers must navigate.
Ineffective Communication and Outreach
Inefficient communication and outreach strategies hinder the ability to reach potential participants. Traditional methods may not be sufficient to engage diverse demographics, and the lack of personalised communication can lead to disinterest or a lack of understanding about the trial. Overcoming this challenge requires innovative approaches to reach a broader audience and convey information in a compelling and accessible manner.
Solutions to Enhance Recruitment and Enrolment:
Digital Marketing and Social Media Campaigns
Leveraging digital platforms for targeted marketing campaigns can enhance outreach efforts. Social media, in particular, offers a powerful tool for reaching diverse demographics. Tailored campaigns that address common concerns, provide educational content, and feature testimonials can create a more engaging and informative environment, encouraging potential participants to consider enrolment.
Patient-Centric Platforms and Mobile Apps
Developing patient-centric platforms and mobile applications can streamline the recruitment process. These platforms can provide a user-friendly interface for individuals to explore trial details, check eligibility, and express interest in participation. Mobile apps can also facilitate real-time communication, allowing researchers to address queries promptly and maintain ongoing engagement.
Community Involvement and Advocacy
Digital platforms can be harnessed to build and strengthen community involvement and advocacy. Creating online forums, discussion groups, and virtual community events can foster a sense of community among potential participants. This not only addresses concerns and builds trust but also encourages word-of-mouth referrals, leveraging the power of social networks to expand outreach.
Overcoming the challenges in recruitment and enrolment in clinical trials necessitates a shift towards digital solutions that prioritise effective communication, community engagement, and targeted outreach. By embracing innovative digital strategies, the clinical research community can enhance participant recruitment, ultimately contributing to the success of trials and the advancement of medical knowledge.
Retention - Navigating Challenges for Long-Term Engagement
Patient retention is a critical aspect of clinical trials, ensuring that participants remain actively involved throughout the study duration. Despite successful enrolment, approximately 20% of patients drop out of clinical trials, presenting a significant challenge to the integrity and reliability of study results. Understanding the reasons behind this attrition and exploring digital solutions is crucial for maintaining participant engagement and obtaining meaningful data.
Challenges in Patient Retention:
Logistical Challenges and Burden on Participants
A primary challenge in patient retention is the logistical burden placed on participants. Frequent clinic visits, complex protocols, and travel constraints can lead to a sense of inconvenience and fatigue among participants. Balancing the need for data collection with minimizing the logistical challenges faced by participants is a delicate yet crucial aspect of retention.
Lack of Communication and Engagement
Once enrolled, participants may feel a lack of ongoing engagement and communication from the research team. This can lead to a sense of disconnect and disinterest, ultimately contributing to dropout rates. Maintaining regular communication and providing updates on trial progress can foster a sense of inclusion and investment in the study.
Unforeseen Side Effects or Lack of Efficacy
Participants may withdraw from a clinical trial due to unexpected side effects or a perceived lack of efficacy in the treatment being studied. This challenge highlights the importance of clear and transparent communication regarding potential risks and benefits, as well as managing participant expectations throughout the trial.
Solutions to Enhance Patient Retention:
Telehealth and Virtual Visits
Integrating tele-health services and virtual visits can alleviate the logistical challenges faced by participants. Conducting certain aspects of the trial remotely, such as follow-up consultations and data collection, reduces the need for frequent in-person visits. This not only enhances participant convenience but also allows for real-time monitoring of health status.
Mobile Apps for Participant Engagement
Developing mobile applications specifically designed for clinical trial participants can enhance engagement and communication. These apps can provide personalised schedules, reminders for upcoming visits or medication doses, and educational content about the trial. Additionally, interactive features such as secure messaging platforms can facilitate direct communication between participants and research teams.
Participant Support Groups and Online Communities
Establishing virtual participant support groups and online communities can create a sense of community among trial participants. These platforms provide a space for participants to share experiences, ask questions, and receive support from both peers and the research team. Building a sense of camaraderie can contribute to a more positive trial experience and increase the likelihood of participants staying engaged.
Addressing the challenges of patient retention in clinical trials requires a proactive and participant-centric approach. Leveraging digital solutions such as tele-health services, mobile apps, and online communities can significantly enhance the overall participant experience, reducing logistical burdens and fostering ongoing engagement. By embracing these innovative approaches, researchers can mitigate dropout rates and ensure the success of clinical trials in advancing medical knowledge and improving patient care.
Conclusion
Effectively navigating the engagement and recruitment process in clinical trials requires a multifaceted approach. From raising awareness and building trust during patient identification to addressing recruitment hesitancy and ensuring participant retention, each stage poses unique challenges. By implementing innovative communication strategies, leveraging community involvement, and adapting trial designs to be participant-centric, researchers can enhance the overall success of clinical trials, advancing medical knowledge and improving patient outcomes. Embracing these technological advancements will not only make clinical trials more efficient and cost-effective but also accelerate the development of life-saving treatments for patients in need. The industry must seize the opportunities presented by digital innovation to ensure a brighter and healthier future for all.



