Engaging, informative, and optimised for Participant Recruitment
Study Website for Recruitment
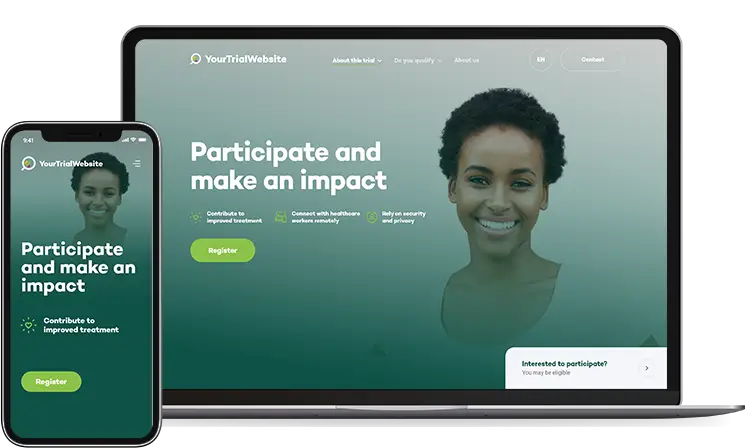
Your Research offers a customisable Study Website module designed to maximise participant recruitment by providing a clear, accessible, and engaging online presence for your clinical study.
This module serves as a central hub where potential participants can learn about the study, complete pre-screening, and even begin the enrolment process—all within a secure, compliant environment.
A dedicated study website increases participant access to study specific information, ensuring a wide-reaching recruitment tool that can attract diverse participants.
This can be shared across various digital channels.
Built-in pre-screening and registration forms allow only eligible participants to proceed, optimizing the recruitment process and reducing administrative workload.
Educational content and multimedia help participants fully understand the study details, building trust and engagement from the start.

Key features
Optimised for Participant Recruitment
The Study Website module by Your Research provides an effective, user-friendly solution for recruitment, giving participants a clear, streamlined path to joining your clinical study while supporting sites with an efficient, secure, and compliant recruitment solution.
Tailor the website’s design, branding, and content to match the study’s requirements and visual identity, creating a professional, trustworthy appearance that resonates with participants.
Offer participants an engaging experience with videos, infographics, FAQs, and other multimedia to explain study goals, procedures, and benefits. This ensures participants are well-informed and feel confident about potential participation.
Partner with recruitment agencies that conduct targeted campaigns to reach specific participant demographics, ensuring a tailored approach to recruitment that aligns with study goals and enhances participant diversity.
Integrate a pre-screening questionnaire and sign-up forms directly on the website, allowing interested participants to check eligibility and register in one seamless process, saving time for both the site and the participants.
Ensure that all personal data entered through the website is securely stored in a compliant, region-specific database, protecting participant privacy and meeting regulatory standards.
The Study Website integrates with the Pre-Consent and Visit Management modules, providing participants with a cohesive journey from initial interest to scheduling their first visit and beyond. This integration simplifies coordination for the site and ensures participants remain engaged and informed.
Connect with other modules
Your Research provides a range of modules beyond Document Management, including CTMS, IRT, ePRO, eCOA, and patient engagement tools. These integrate seamlessly to create a unified platform, streamlining trial management, enhancing data accuracy, and improving participant engagement and retention.
