Empowering sites to achieve seamless operations and drive trial success
Site Engagement
At Your Research, we see sites as a crucial element for trial success. Supported by our workflow-driven solutions, sites can streamline Visit Management, enhance Patient Engagement, and follow a clear, protocol-driven roadmap for each required action.
We prioritise efficiency and quality of trial operations by reducing administrative burdens and ensuring strict protocol adherence.
By incorporating essential third-party systems like EDC, eCOA, eTMF, and IRT—or utilising our own modules—Your Research guides sites on which systems to use at each stage, enhancing data consistency and workflow clarity. This seamless integration of key vendors boosts site performance, aligns every action with study protocols, reduces errors, and raises overall quality.

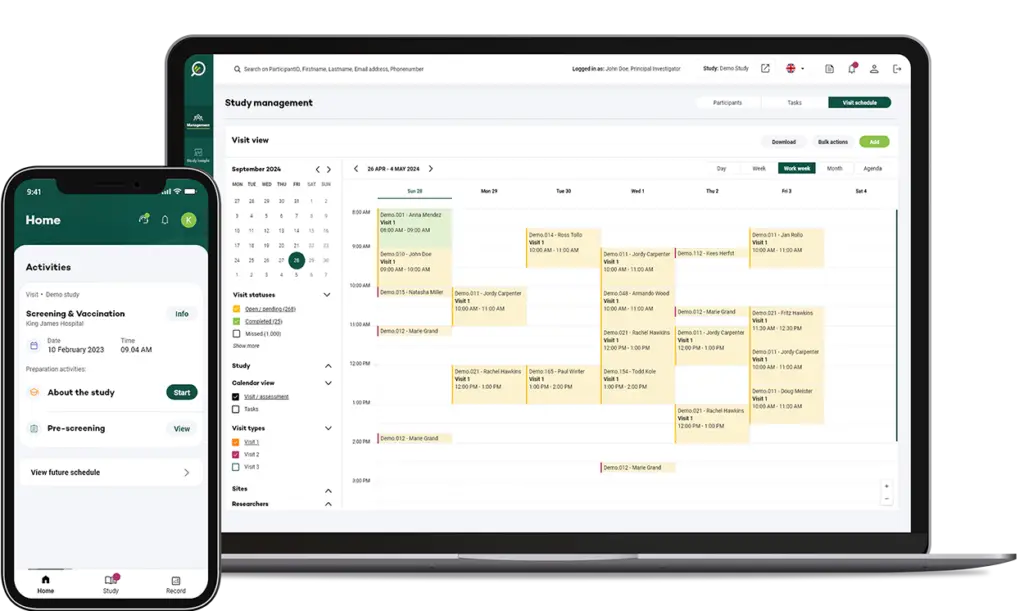
Visit Management: Simplifying Protocol-Driven Visits
Our unique Visit Management module makes it easy to schedule visits according to protocol requirements with a built-in forecasting tool that alerts you when an upcoming action may be missed.
It supports both automated and manual scheduling, depending on visit type, and is directly linked to available resources for seamless coordination.
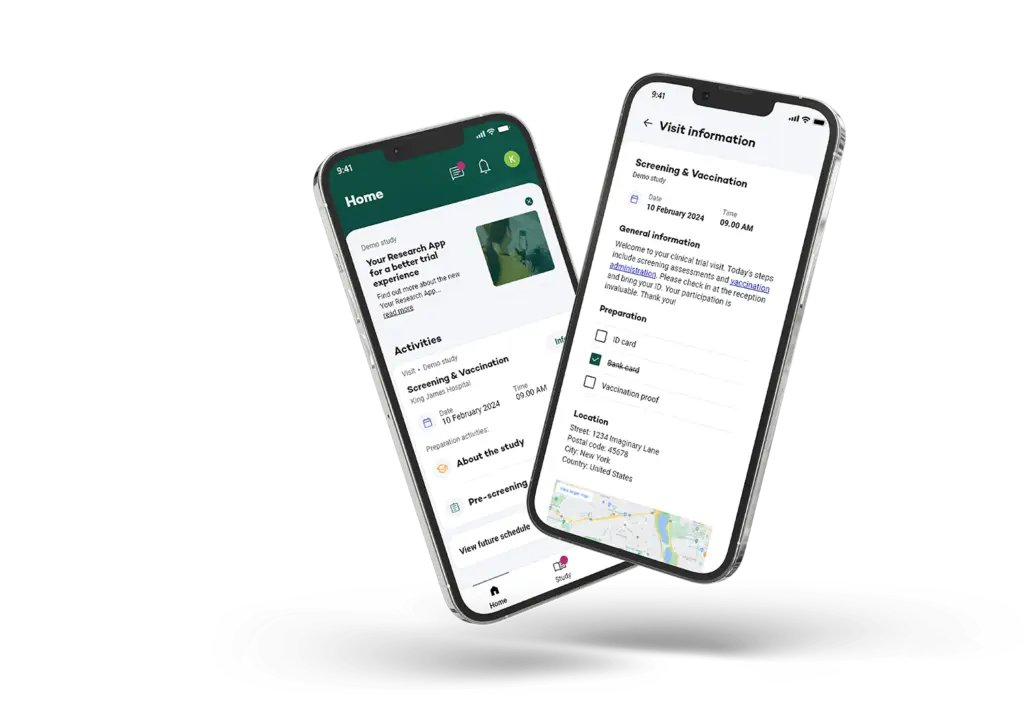
Patient Engagement: Enhancing the Participant Journey
Our solution simplifies participant engagement through a multi-channel approach, allowing participants to stay connected via their preferred devices, whether on-site, at home, or through a home care setting.
Your Research helps you build meaningful relationships with participants by providing consistent, clear, and engaging interactions throughout the trial.
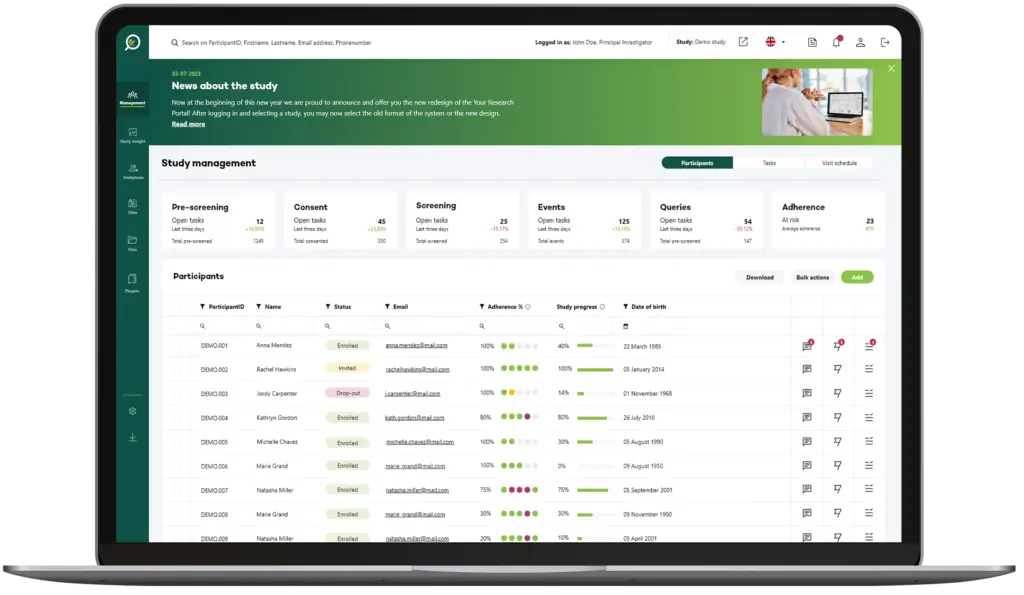
Protocol-Driven Insights: Staying on Track with Ease
With Your Research, sites gain a protocol-driven dashboard that provides immediate insight into the next steps required in a trial, keeping activities on track.
This feature minimises the administrative burden on site teams, reducing protocol deviations and ensuring that each action aligns seamlessly with study requirements.
Modules at glance
Delivering Site Solutions with measurable benefits to Sponsors
Whether as a standalone solution or part of a broader CTMS, Your Research empowers sites to engage participants effectively, manage trials efficiently, and uphold the highest standards of quality throughout the study.
