Empowering Participants and Streamlining Study Enrolment
Pre-Consent
Our ‘Pre-Consent’ Module is designed to simplify the initial stages of clinical trial participation, allowing participants to engage effortlessly while sites manage pre-consent activities efficiently.
With features like participant self registration, pre-screening, and self-scheduling, this module supports compliance, engagement, and data integrity from the very beginning of the Participant Journey.
Integrated registration, pre-screening, and scheduling options minimise delays in study start-up and reduce administrative burden for sites.
Early access to study information and streamlined registration and pre-screening foster a sense of confidence and keep participants informed.
Region-specific data storage and privacy-compliant handling protect personal data and ensure compliance with local regulations.
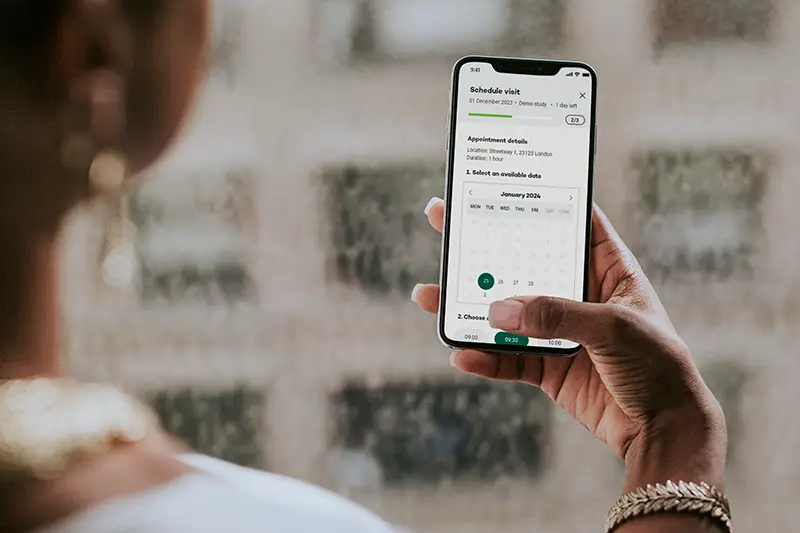
Key features
Enrol participants with ease
With the Pre-Consent Module, Your Research offers a participant-friendly, secure, and efficient pre-consent process that lays a strong foundation for successful trial participation.
Allow participants to register through an easy-to-use sign-up form, complete initial pre-screening questions, and understand eligibility early. This streamlines the recruitment process, ensuring that only eligible participants proceed further.
Participants can schedule their own appointments, choosing a time that fits their availability. This feature, also available in our Visit Management module, empowers participants to take control of their journey and reduces site coordination time.
The Pre-Consent module integrates seamlessly with your website or our Study Website module, ensuring participants have consistent, accurate information and a smooth journey from the public website to the pre-consent st
All participant data is securely stored in a compliant, region-specific database to ensure data protection and privacy across global regulations. Built-in tracking tools also record each participant interaction for a transparent, audit-ready process.
Offer participants multiple ways to complete the pre-consent process, including online, mobile, or in-person options. This flexibility accommodates participant preferences, ensuring accessibility for all.
Sites have access to real-time updates on each participant’s pre-consent status, allowing coordinators to monitor progress, send reminders, and assist as needed, improving enrolment efficiency.
Connect with other modules
Your Research provides a range of modules beyond Document Management, including CTMS, IRT, ePRO, eCOA, and patient engagement tools. These integrate seamlessly to create a unified solution, streamlining trial management, enhancing data accuracy, and improving participant engagement and retention.
