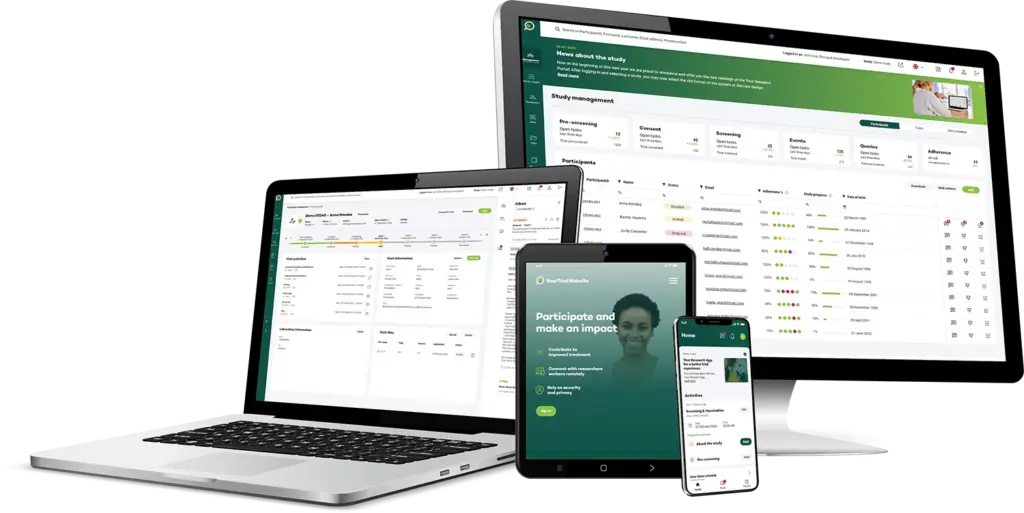
Revolutionizing Patient Data Management
Patient Registry
Your Research’s Patient Registry is designed to transform how clinical data is collected and managed. By providing an all-in-one, secure solution, we empower research teams to streamline their data processes, enhance participant engagement, and drive impactful research outcomes.
Streamline the collection, organization, and analysis of patient data to enhance research accuracy and efficiency.
Maintain confidence with a platform that meets strict international data privacy standards, ensuring patient information is protected.
Adapt the registry to various therapeutic areas, from oncology to cardiology, for versatile and precise data management.

Key features
Comprehensive Solutions for Clinical Excellence
The Your Research Patient Registry is built with a suite of powerful features aimed at supporting research teams and ensuring the successful execution of clinical studies. With user-centric design and flexible functionality, this solution is tailored to meet modern research demands.
Simplifies data input and navigation, making it easier for research teams to manage patient information with minimal training.
Seamlessly connects with electronic Patient-Reported Outcomes and Clinical Outcome Assessments to provide a holistic view of patient progress and results.
Minimise administrative workload with automated notifications, updates, and task management, allowing research teams to focus on key analytical tasks.
The registry’s adaptable framework allows for personalised configurations tailored to different research protocols and therapeutic areas.
Advanced data protection measures ensure that all patient information is encrypted and managed according to international regulatory standards, fostering trust and safety.