Streamlining Consent in modern clinical trials
eConsent
Your Research’s eConsent is designed to simplify and enhance the electronic and paper-based consent process for clinical trials. With eConsent, you can ensure compliance, improve participant understanding, and streamline the consenting process, making it easier for both researchers and participants.
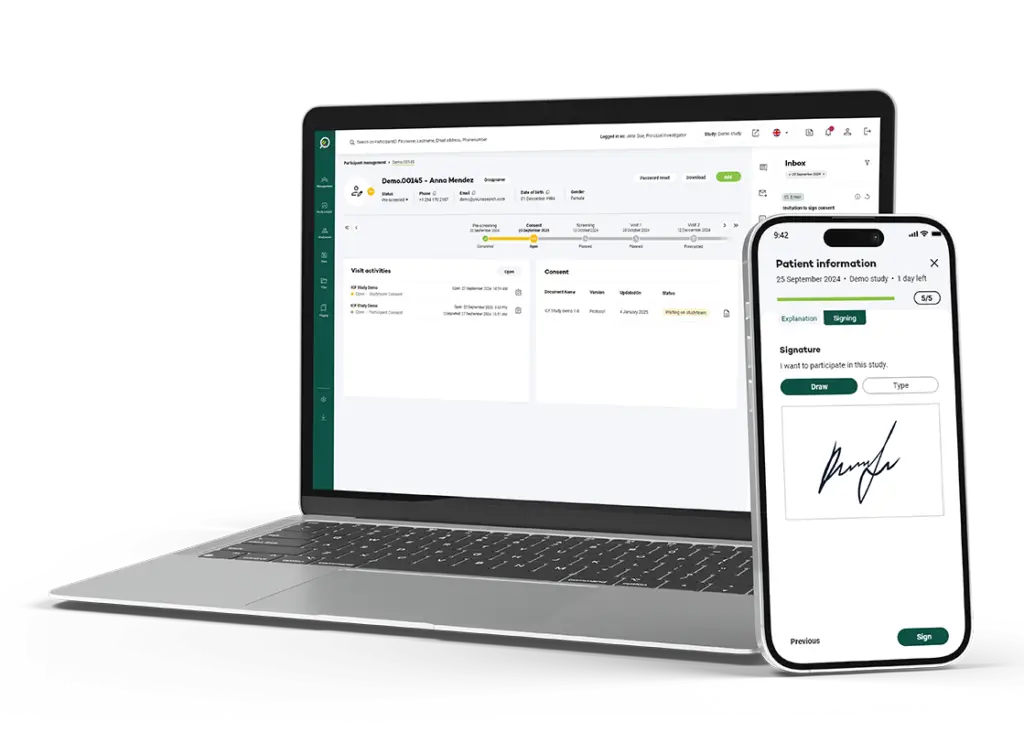
Meet regulatory requirements with secure electronic signatures that include audit trails, ensuring transparency and compliance throughout the entire process.
Effortlessly track participant consent status with a user-friendly dashboard that provides real-time updates, ensuring you stay informed of the latest developments.
Our platform features an intuitive design that is easy to navigate, enabling participants of all ages to engage seamlessly and confidently in the process.

Key features
Ensure compliance, improve participant understanding
Experience the ultimate flexibility of our modular eConsent platform for clinical trials. Tailor it to your study protocol, ensuring a seamless and customised consent process that aligns perfectly with your research objectives.
Enhance the consent process for participants by incorporating interactive features and multimedia elements such as questionnaires and assessments to ensure comprehension and enable participants to make informed decisions about their participation.
Conveniently engage remotely to assess eligibility and allow prospective participants to ask questions via remote video call or chat. Or simply use Your Research's eConsent module as an efficient aid in your face to face interaction.
Sign with an advanced (AES) or qualified electronic signature (QES) a consent form offers the benefit of convenience, as it eliminates the need for physical paperwork and allows participants to easily review and sign the document in a secure environment from anywhere, streamlining the process and potentially expediting their involvement in the study.
Streamline the re-consent process in clinical trials by enabling instant, compliant updates and remote participant re-approval, reducing delays and ensuring all participants are informed of protocol changes in real time.
Our services
Streamline service delivery to minimize risks and expedite startup
Minimising startup and delay risks is the goal of our services. Whether you prefer a hybrid or full self-service model, we are ready to assist with our expertise and experience, ensuring peace of mind.
Our network of translators will support with validated translation of questionnaires and communication information for participants.
Together with our technology, local partners and pre-defined documentation, we support you to have oversight into your IRB approval process.
We provide 24/7 assistance to address technical issues and user inquiries, as well as training for clinical staff to ensure they are proficient in using Your Research.
Modules at glance
Elevate the whole Participant Journey
Serving as the essential foundation, our Unified Protocol enhances trial outcomes and facilitates the seamless integration of both modules and third-party systems along the Participant Journey.
This foundational structure ensures comprehensive oversight and enables strategic foresight throughout the patient experience.





