Real-time, accurate, and compliant data collection at the Source
eSource
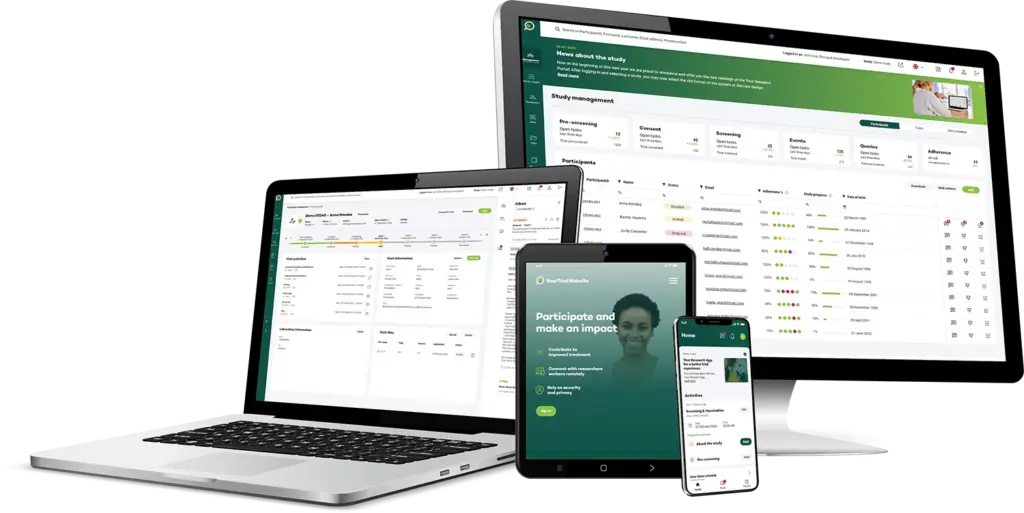
Your Research’s eSource module provides an efficient, secure solution for capturing data directly from the source, transforming clinical trials with real-time data collection, enhanced data integrity, and seamless integration into the clinical trial management system. By digitising the process, eSource minimises data entry errors, reduces administrative burden, and ensures that all data is traceable, compliant, and audit-ready.
Direct data capture minimises transcription errors, reduces the need for manual entry, and ensures that data is complete and accurate from the start.
Automated integration with eCRF and CTMS reduces administrative burden on site staff, allowing them to focus on participant care and study oversight.
With real-time audit trails, data validation, and secure storage, eSource ensures that all data is compliant, traceable, and always ready for audits and inspections.

Key features
Empowering data accuracy with eSource
Your Research’s eSource module is an essential tool for modern clinical trials, providing accurate, real-time data capture that aligns with regulatory standards and enhances the quality and efficiency of trial data management. By digitising source data collection, eSource supports faster, more compliant trials that lead to reliable, high-quality outcomes.
Capture data at the point of care in real-time, allowing for immediate recording of participant information, observations, and other critical trial data. This real-time capability enhances data accuracy and provides timely insights
eSource data integrates seamlessly with electronic Case Report Forms (eCRF) and the Clinical Trial Management System (CTMS), ensuring that all captured data flows directly into the study database without the need for duplicate entry or manual transfers.
Our eSource module maintains a complete audit trail for all entries, modifications, and corrections, supporting data integrity and making the data fully traceable, ensuring it meets regulatory and protocol standards.
Built-in data validation rules help ensure data accuracy at the point of entry, alerting users to potential errors or inconsistencies immediately to reduce the need for costly data cleaning later on.
Collect a variety of data types, from text entries to images, videos, and measurements from integrated devices. This flexibility allows sites to capture a full range of information, ensuring robust data capture that aligns with study protocols.
All eSource data is stored securely and in compliance with global regulatory standards, including ICH-GCP, FDA, and EMA. Data is protected based on regional regulations, ensuring privacy and security for participants.
Connect with other modules
Your Research provides a range of modules beyond Document Management, including CTMS, IRT, ePRO, eCOA, and patient engagement tools. These integrate seamlessly to create a unified solution, streamlining trial management, enhancing data accuracy, and improving participant engagement and retention.
