Optimizing Clinical Trial Visit Management
Visit Management
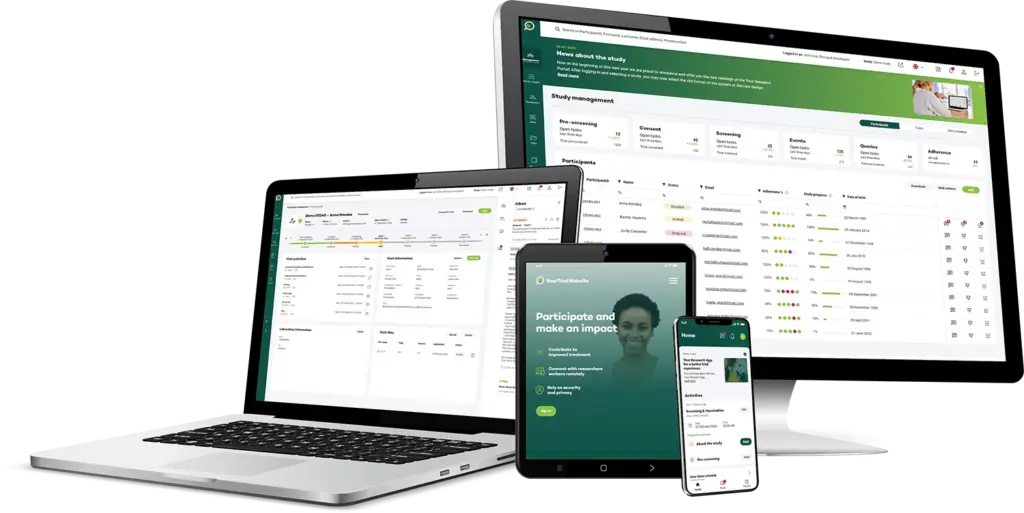
Your Research’s Visit Scheduler is designed to simplify and optimise the scheduling of participant and monitor visits for clinical trials.
This tool ensures efficient coordination, reduces administrative burden, and enhances participant compliance and experience, making the management of clinical trials more streamlined and effective.
This comprehensive approach to visit management benefits both sites and sponsors by reducing administrative strain, enhancing protocol adherence, and improving the overall quality of trial management.
Improve participant retention through robust visit preparation, automated and personlised reminders and a give overview via a mobile application (App).
Streamline visit scheduling and reduce the administrative workload with automated processes., such as automated reminders and resource planning.
Reduce protocol deviations based on prospective insights and mitigate participant retention risks.

Key features
Simplify protocol driven visits with comprehensive trial oversight
Our unique Visit Management module makes it easy to schedule visits according to protocol requirements, with a built-in forecasting tool that alerts you when an upcoming action may be missed.
Supporting both automated and manual scheduling based on visit type, this module is directly linked to available resources for seamless coordination across all visit types, including participant, investigator, and monitor visits.
Easily plan visits based on protocol specifications, with options for both automated and manual scheduling to suit the complexity of each visit type
Link schedules to site resources, providing a unified overview of trial activities across multiple studies and teams, ensuring that all visits—whether for participants or monitors—are efficiently organised.
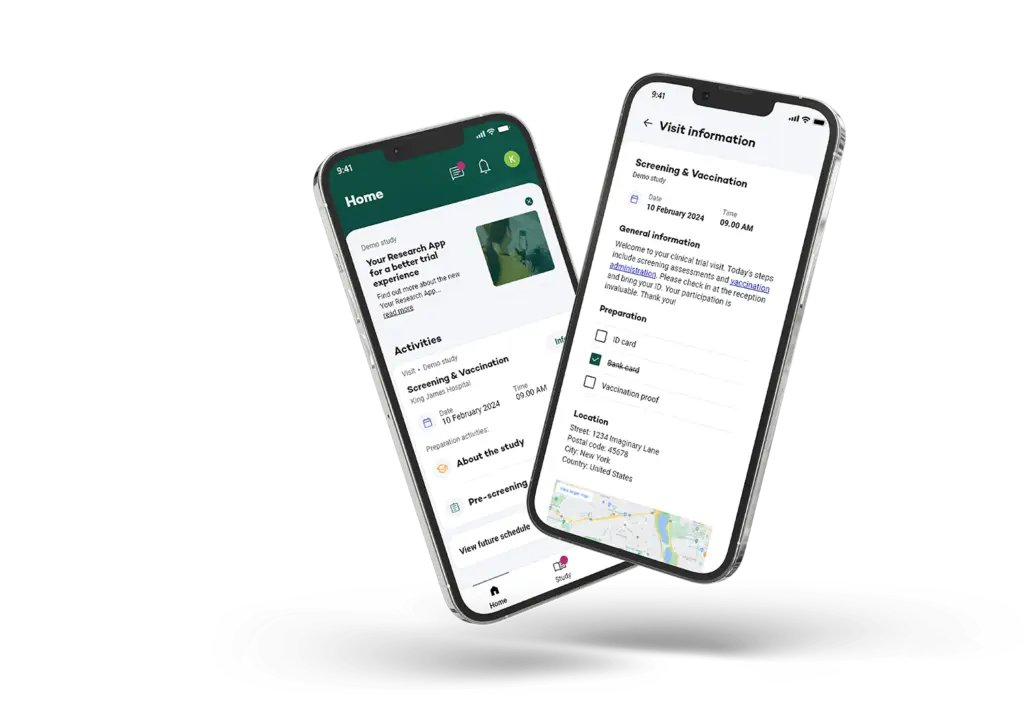
Send reminders via email or SMS to participants and site staff, with the option to add multimedia for a more engaging experience.
Allow participants to select convenient visit times based on resource availability, giving them greater control over their participation.
Add video call links to visit plans, supporting remote visits that increase accessibility and scheduling flexibility for participants, site staff, and monitors alike.
Schedule and manage monitor visits with ease, ensuring that monitors have access to the resources and data they need while staying aligned with protocol timelines.
Support of different types of Visits
Easily set up different types of visits by pre-defining your availability.
On-site visit
Home care visit
Tele visit
Phone call visit
Monitor visit
Key benefits
Improve protocol adherence and efficiency
Ensure efficient coordination, reduce site administrative burden, and enhance participant compliance. Making the management of clinical trials more streamlined and effective, resulting in high-quality data.
Visit Management ensures all visits are scheduled according to protocol requirements, with built-in forecasting and alerts for upcoming tasks. This reduces protocol deviations and keeps studies on track, supporting compliance and enhancing trial quality.
By integrating with site resources, this module allows for smart scheduling across multiple studies and visit types. Automated scheduling options and resource linking streamline visit coordination, saving time and reducing administrative burden for site teams.
With options for automated reminders, multimedia notifications, and participant self-scheduling, this module supports a smooth and engaging participant experience. It accommodates diverse visit types—on-site, home care, tele-visits, and phone calls—allowing participants to choose options that best suit their needs.